
Concept explainers
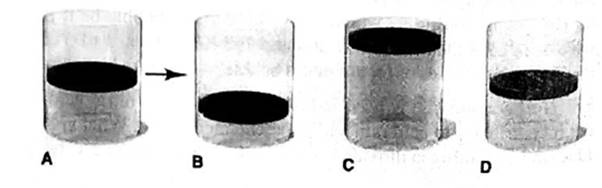
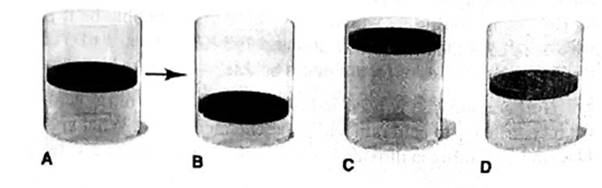
In A, the picture shows a cylinder with 0.1 mol of a gas that behaves ideally. Choose the cylinder (B, C, or D) that correctly represents the volume of the gas after each of the foflowing changes. If none of the cylinders is correct, specify “none.”
(a) P is doubled at fixed n and T
(b) T is reduced Ironi 400 K to 200 K at fixed n and P.
(c) T is increased from 100°C to 200°C at fixed is and P
(d) 0.1 mol of gas is added at fixed P and T.
(e) 0.1 mol of gas is added and P is doubled at fixed T.
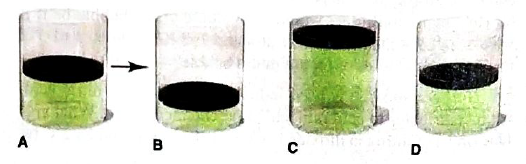
(a)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when the pressure of a gas is doubled at fixed number of moles and temperature.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
At constant temperature and number of moles of a gas, the volume of a gas is ½ of the initial volume when the pressure of a gas is doubled.
Thus, it is represents by Cylinder B.
Explanation of Solution
Given information:
The cylinders are:
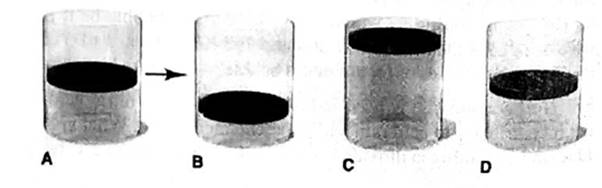
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The new ideal expression is shown below, when the pressure is doubled at constant number of moles and temperature.
Now, the new volume is calculated as:
Thus, new volume is:
Hence, at constant temperature and number of moles of a gas, the volume of a gas is ½ of the initial volume when the pressure of a gas is doubled.
Thus, it is represents by Cylinder B.
(b)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when the temperature of a gas is reduced from 400 K to 200 K at constant number of moles and pressure.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
The final volume of a gas is ½ times of the initial volume of a gas and it is described by the Cylinder B.
Explanation of Solution
Given information:
The cylinders are:
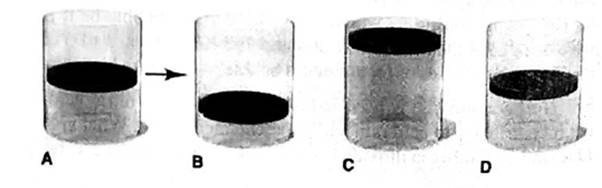
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The ideal gas law for two given conditions is:
Where,
Put the values,
Thus, the final volume of a gas is ½ times of the initial volume of a gas and it is described by the Cylinder B.
(c)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when the temperature of a gas is increased from 100
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
The final volume of a gas is 1.26 times of the initial volume of a gas. It is described by “None” cylinder.
Explanation of Solution
Given information:
The cylinders are:
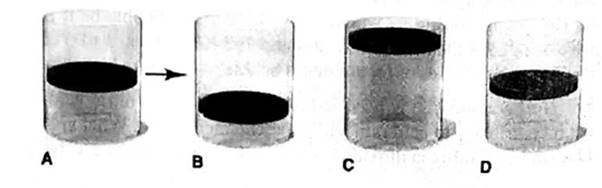
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The ideal gas law for two given conditions is:
Where,
Convert the value of temperature in degree Celsius in Kelvin.
Initial temperature in K =
Final temperature in K =
Put the values,
Thus, the final volume of a gas is 1.26 times of the initial volume of a gas and it is not described any cylinder.
(d)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when 0.1 mole of a gas is added at constant temperature and pressure.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
At constant temperature and pressure of a gas, the volume of a gas is 2 times of the initial volume when the 0.1 mole of a gas is added.
Thus, it is represents by Cylinder C.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The new ideal expression is shown below, when 0.1 mole of a gas is added at constant temperature and pressure.
Now, the new volume is calculated as:
Thus, new volume is:
Hence, at constant temperature and pressure of a gas, the volume of a gas is 2 times of the initial volume when the 0.1 mole of a gas is added.
Thus, it is represents by Cylinder C.
(e)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when 0.1 mole of a gas is added and Pressure is doubled at constant temperature.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
At constant temperature of a gas, the volume of a gas is equal to the initial volume when the 0.1 mole of a gas is added and pressure is doubled.
Thus, it is represents by Cylinder D.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The new ideal expression is shown below, when 0.1 mole of a gas is added and Pressure is doubled at constant temperature
Now, the new volume is calculated as:
Thus, new volume is:
Hence, at constant temperature of a gas, the volume of a gas is equal to the initial volume when the 0.1 mole of a gas is added and pressure is doubled.
Thus, it is represents by Cylinder D.
Want to see more full solutions like this?
Chapter 5 Solutions
Principles of General Chemistry
- 5-107 If 60.0 g of NH3 occupies 35.1 L under a pressure of 77.2 in. Hg, what is the temperature of the gas, in °C?arrow_forwardperform stoichiometric ca1cu1uions for reactions involving gases as reactants or products.arrow_forwardIn the Mthode Champenoise, grape juice is fermented in a wine bottle to produce sparkling wine. The reaction is C6H12O6(aq)2C2H5OH(aq)+2CO2(g) Fermentation of 750. mL grape juice (density = 1.0 g/cm3) is allowed to take place in a bottle with a total volume of 825 mL until 12% by volume is ethanol (C2H5OH). Assuming that the CO2 is insoluble in H2O (actually, a wrong assumption), what would be the pressure of CO2 inside the wine bottle at 25C? (The density of ethanol is 0.79 g/cm3.)arrow_forward
- 5-39 An 8.00-g sample of a gas occupies 22.4 L at 2.00 atm and 273 K. What is the molar mass of the gas?arrow_forward5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous acid to calcium carbonate based on the following balanced net ionic equation: (a) How many moles of wet CO (g), collected at 60.°C and 774 torr total pressure, are produced by the complete reaction of 10.0 g of CaCO3 with excess acid? (b) What volume does this wet CO2 occupy? (c) What volume would the CO2 occupy at 774 torr if a desiccant (a chemical drying agent) were added to remove the water? The vapor pressure of water at 60.°C is 149.4 mm Hg.arrow_forward5-25 A gas in a bulb as in Figure 5-3 registers a pressure of 833 mm Hg in the manometer in which the reference arm of the U-shaped tube (A) is sealed and evacuated. What will the difference in the mercury levels be if the reference arm of the U-shaped tube is open to atmospheric pressure (760 mm Hg)?arrow_forward
- A 2.0 L soda bottle is pressurized with 4.5 atm of CO2 at 298 K. If the temperature is increased to 317 K, what is the pressure of the CO2?arrow_forwardA typical barometric pressure in Kansas City is 740 torr. What is this pressure in atmospheres, in millimeters of mercury, and in kilopascals?arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





