
Concept explainers
(a)
Interpretation:
The condensed structural formula and systematic name should be given for the molecular formula of
Concept introduction:
A condensed structural formula is a system of writing organic structures in a line of text.
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Constitutional Isomers: A molecule having same molecular formula with different structural formulas (Difference in the connectivity of the molecule is called constitutional isomer).
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry).IUPAC name consists of three parts, namely Prefix, suffix and root word.
Prefix- Represents the substituent present in the molecule and its position in the root name.
Suffix- Denotes the presence of
Root word - Represents the longest continuous carbon skeleton of the organic molecule.
Alkenes are a class of hydrocarbons. The carbon-carbon double bond is called as alkenes and it is also called as olefins.
(b)
Interpretation:
The E and Z isomer of the alkene has to be identified.
Concept introduction:
E and Z isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:
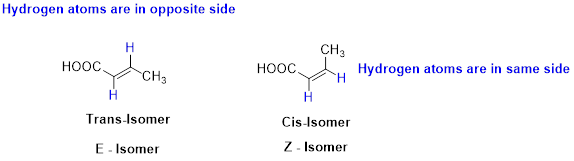
(c)
Interpretation:
The most stable alkene has to be identified.
Concept Introduction:
Stability of alkene:
Stability of alkene depends on the following factors,
The smallest heat hydrogenation of alkene is more stable. The number of hydrogens bonded to its
Stability of Cis–trans alkene:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
In cis alkene, molecule are close to each other, hence electron clouds interfere each other therefore strain in the molecule so cis alkene is less stable. Whereas molecule is away from each other, hence electron clouds are will not interfere each other therefore less strain in the molecule, so trans alkene is stable.
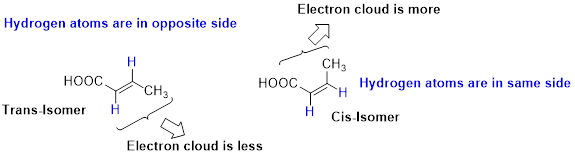
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Essential Organic Chemistry (3rd Edition)
- 2. Draw and name four alkenes with the formula C,Hg.arrow_forward1. An alkane, P, has the molecular formula, CoHv. An Alkene, Q, has the molecular formula, GHs. a) Name P and Q and write their full structural formula. b) State two differences between P and Q in terms of their structures.arrow_forward1. Below is the structure of a carbon atom, how is this structure related to the formation of varied forms of organic compounds? a. By attracting other elements toward themselves to form the bonds. b. By forming may bonds with other carbon atoms and other elements. c. By sharing their electrons with other metal and non-metal elements. d. By transferring their electrons to the atoms of surrounding e Protons C6 Neutrons 6 Electrons elements 2. Below is the structure of a sample hydrocarbon, how many types of bond are present in the sample hydrocarbon? H а. 1 b. 2 H C C н с с (C3H4) H с. d. 4 Harrow_forward
- Organicarrow_forward7. C=C. A hydrocarbon that contains a -C=C- or the above group. Oa. addition reaction Ob. aliphatic compound C. alkene Od. alkyne e. aromatic hydrocarbon f. hydration g. hydrogenation h. monomer i. phenyl group j. polycyclic aromatic hydrocarbon Ok. polymer O1. unsaturated hydrocarbonarrow_forward6. Consider the following reactions: A. When CH12 is reacted with C2(g) in the presence of ultraviolet light, four different monochlorination products form. What is the structure of CsHh2 in this reaction? B. When CaH is reacted with H20, a tertiary alcohol is produced as the major product. What is the structure of C4H, in this reaction?arrow_forward
- Name the given formulas.arrow_forwarda. How many Lewis structures have the formula C4H11N? 10 b. In how many of the structures is the nitrogen atom attached to only one carbon? c. How many of them have carbon-carbon double bonds? d. How many of them have carbon-nitrogen double bonds? e. How many of them have rings? f. How many of the structures are capable of hydrogen bonding? g. How many of the structures contain a carbon atom attached to three other carbons? h. How many of the structures contain a nitrogen atom attached to three carbons? Varrow_forwardGive small explanation.arrow_forward
- .Acetylene molecule C2H2 is - - 47 A -Polar O B - Not polar Cycloalkanes are alkanes that - 48 .have carbon atoms that form A- A ring (called alicyclic compounds). O B - Aromatic compounds. with Br2 to yield - 49 .an addition product A Does not react B - React. .The unit of density is - 50 A - g/cm2. BKm/ cm. C - g/cm3. Benzene *********arrow_forward1. Give IUPAC names for each of the following alkenes: a. b. C. d. e. CH3 -CH₂ C - CH₂ CH₂ - CH3 || CH₂ CICH=CHCH3 CH3 -CH3 -CH(CH3)2 2. Draw structures corresponding to the following IUPAC names: a. 2-Methylhex-1-ene b. (3Z, 6E)-1,3,6-octatriene c. vinylcyclopropanearrow_forwardThe alkene 3-methyl-1-butene is reacted with Br₂ in aqueous solution. Br₂. H₂Oarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


