
Concept explainers
For each of the following molecules or ions that contain sulfur, write the Lewis structure(s), predict the molecular structure (including bond angles), and give the expected hybrid orbitals for sulfur.
a. SO2
b. SO3
c. 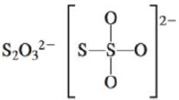
d. 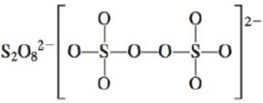
e. SO32−
f. SO42−
g. SF2
h. SF4
i. SF6
j. F3S—SF
k. SF5+
(a)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on each sulfur and oxygen atom. Two oxygen atoms are bonded to sulfur atom. Therefore, the total valence electrons are
Therefore the geometry is bent. The bond angle is less than
The Lewis structure of
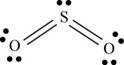
Figure 1
(b)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Two oxygen atoms are attached to sulfur, therefore, the total number of valence electrons is
The molecule has trigonal planar geometry with bond angle
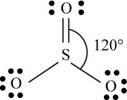
Figure 2
(c)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Three oxygen atoms and one sulfur atom is attached to central sulfur atom and charge on the molecule is
By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
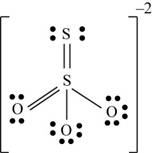
Figure 3
(d)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. There are eight oxygen atoms and two sulfur atoms are present in the molecule and charge on the molecule is
The two oxygen atoms in the centre are bonded by single bond. By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
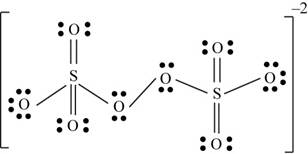
Figure 4
(e)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Three oxygen atoms and one sulfur atom present in the molecule and charge on the molecule is
One oxygen atom is single bonded with sulfur and one is joined by pi bond. By bonding in this way, they complete their octet. The molecular structure is trigonal pyramidal with bond angle approximately equal to
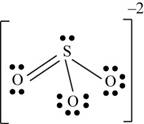
Figure 5
(f)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Four oxygen atoms and one sulfur atom is present in the molecule and charge on the molecule is
Two oxygen atoms are single bonded with sulfur and two joined by pi bond. By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
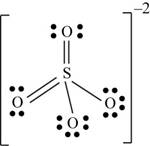
Figure 6
(g)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Two fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The sulfur is bonded to two fluorine atoms by sigma bond. By bonding in this way, they complete their octet. The molecular structure is bent due to presence of lone pairs of electrons on sulfur. The bond angle is less than
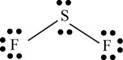
Figure 7
(h)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Four fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The molecular structure is see-saw due to presence of lone pair of electrons on sulfur. The equatorial bond angles are
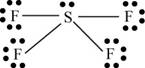
Figure 8
(i)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Six fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The molecular structure is octahedral with bond angle
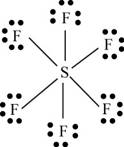
Figure 9
(j)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Four fluorine atoms and two sulfur atoms are present in the molecule, therefore, the total number of valence electrons is
The molecular structure is see-saw due to presence of lone pair of electrons on sulfur. The equatorial bond angles are
The Lewis structure of
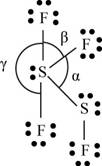
Figure 10
(k)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Five fluorine atoms and one sulfur atom is present in the molecule and charge on the molecule is
The molecular structure is trigonal bipyramidal with equatorial bond angles
The Lewis structure of
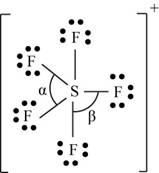
Figure 11
Want to see more full solutions like this?
Chapter 4 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
- Hi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forwardHi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forward
- Briefly explain the following paragraph: both the distortion of symmetry and the fact that the solid is diamagnetic indicate the existence of a Nb-Nb bond.arrow_forwardHi I need help on my practice final, If you could explain how to solve it, offer strategies, and dumb it down that would be amazing.arrow_forward-1 2 3 4 5 7 8 At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0635 s 2C1,0, (g) →2C1, (g)+50, (g) Suppose a vessel contains C1,0, at a concentration of 1.03 M. Calculate how long it takes for the concentration of C1,0, to decrease by 86.0%. You may assume no other reaction is important. Round your answer to 2 significant digits. e х th Earrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





