
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
15th Edition
ISBN: 9781118930144
Author: Willard
Publisher: JOHN WILEY+SONS INC.
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 45CE
(a)
Interpretation Introduction
Interpretation:
The change occurred in the illustration has to be given.
The given diagram is,
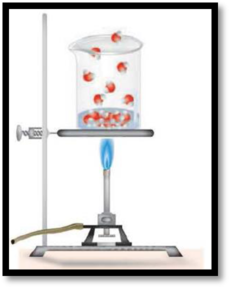
Figure 1
(b)
Interpretation Introduction
Interpretation:
The change occur in the illustration is physical or chemical change has to be given.
The given diagram is,
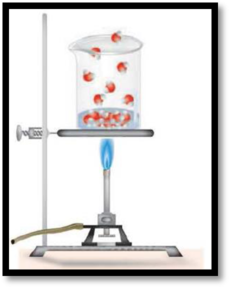
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You heat 2.53 grams of metallic mercury in air, which produces 2.73 grams of red-orange residue. Assume that the chemical change is the reaction of the metal with oxygen in the air.
What is the mass of oxygen that react? When you strongly heat the red- orange residue, it decomposes to give her back the mercury and release the oxygen, which you collect. What is the mass of oxygen you collected?
There are four sketches below. The first sketch shows a sample of Substance X. The three sketches underneath it show three different changes to the sample.
You must decide whether each of these changes is possible. If a change is possible, you must also decide whether it is a physical change or a chemical change.
Each sketch is drawn as if the sample were under a microscope so powerful that individual atoms could be seen. Also, you should assume that you can see the
entire sample, and that the sample is in a sealed box, so that no matter can enter or leave.
Sample of Substance X
Change 1
Change 1 is
O impossible
O
O a chemical change
a physical change
Change 2
Change 2 is:
impossible
O a physical change
O a chemical change
Change 3
000 000
Change 3 is:
O impossible
O a physical change
O a chemical change
X
How do I figure this out?
Chapter 4 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
Ch. 4.1 - Prob. 4.1PCh. 4.2 - Prob. 4.2PCh. 4.5 - Prob. 4.3PCh. 4.5 - Prob. 4.4PCh. 4.5 - Prob. 4.5PCh. 4 - Prob. 1RQCh. 4 - Prob. 2RQCh. 4 - Prob. 3RQCh. 4 - Prob. 4RQCh. 4 - Prob. 5RQ
Ch. 4 - Prob. 6RQCh. 4 - Prob. 7RQCh. 4 - Prob. 8RQCh. 4 - Prob. 9RQCh. 4 - Prob. 10RQCh. 4 - Prob. 11RQCh. 4 - Prob. 12RQCh. 4 - Prob. 13RQCh. 4 - Prob. 14RQCh. 4 - Prob. 15RQCh. 4 - Prob. 1PECh. 4 - Prob. 2PECh. 4 - Prob. 3PECh. 4 - Prob. 4PECh. 4 - Prob. 5PECh. 4 - Prob. 6PECh. 4 - Prob. 7PECh. 4 - Prob. 8PECh. 4 - Prob. 9PECh. 4 - Prob. 10PECh. 4 - Prob. 11PECh. 4 - Prob. 12PECh. 4 - Prob. 13PECh. 4 - Prob. 14PECh. 4 - Prob. 15PECh. 4 - Prob. 16PECh. 4 - Prob. 17PECh. 4 - Prob. 18PECh. 4 - Prob. 19PECh. 4 - Prob. 20PECh. 4 - Prob. 21PECh. 4 - Prob. 22PECh. 4 - Prob. 23AECh. 4 - Prob. 24AECh. 4 - Prob. 25AECh. 4 - Prob. 26AECh. 4 - Prob. 27AECh. 4 - Prob. 28AECh. 4 - Prob. 29AECh. 4 - Prob. 30AECh. 4 - Prob. 31AECh. 4 - Prob. 32AECh. 4 - Prob. 33AECh. 4 - Prob. 34AECh. 4 - Prob. 35AECh. 4 - Prob. 36AECh. 4 - Prob. 37AECh. 4 - Prob. 38AECh. 4 - Prob. 39AECh. 4 - Prob. 44CECh. 4 - Prob. 45CECh. 4 - Prob. 46CE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- hat do the coefficients of a balanced chemical equation tell us about the proportions in which atoms and molecules react on an individual (microscopic) basis?arrow_forwardWhat is the main difference between electrostatic forces and gravitational forces? Which is more similar to the magnetic force? Can two or all three of these forces be exerted between two objects at the same time?arrow_forward3.83 For the reaction of nitrogen, N2, and hydrogen, H2, to form ammonia, NH3, a student is attempting to draw a particulate diagram, as shown below. Did the student draw a correct representation of the reaction? If not, what was the error the student made?arrow_forward
- Suppose someone emptied ball bearings into a container of salt. Could you separate the ball bearings from the salt? How? Would your method involve no change, be a physical change, or be a chemical change?arrow_forwardIn each case, describe the change as a chemical or physical change. Give a reason for your choice. Salt dissolves when you add it to water. Food is digested and metabolized in your body. Crystalline sugar is ground into a fine powder. When potassium is added to water there is a purplishpink flame and the water becomes basic (alkaline).arrow_forwardParticles in the illustration below undergo a chemical change. Which among the remaining boxes, a through d, can represent the products of the chemical change? If a box cannot represent the products of the chemical change, explain why. a b c darrow_forward
- A sample of solid silver oxide with a mass of 11.4 grams was reduced to elemental silver by heating under a flow of methane gas, CH4. The reaction produced 10.6 grams of silver. Write a balanced chemical reaction for the reaction between silver oxide and methane gas. The only products formed in the reaction are solid silver metal, carbon dioxide gas, and water vapor. Include the phases of each reactant and product.arrow_forwardThe boiling of water is aa. physical change because the water disappears.physical change because the gaseous water is chemically the same as the liquid.chemical change because heat is needed for the process to occur.chemical change because hydrogen and oxygen gases are formed from water.chemical and physical change. Explain your answerarrow_forwardFor each of the changes, decide whether two or more elements formed a compound or If a compound decomposed (to elements or other compounds). Explain your reasoning in each case. a) upon heating, a blue powder turned white and lost mass b) A white soil forms three different gasses when heated. The total mass of the gases is the same as that the solid.arrow_forward
- 4. If the Law of Conservation of Energy states that energy is neither be created nor destroyed, but can only be transferred or changed from one form to another, why do scientists worry about running out of energy in the future?arrow_forward3. The following shows a chemical reaction between carbon dioxide and water to form carbonic acid: carbon dioxide + water > carbonic acid COo + H20 > H;CO; Based on the given chemical reaction as an example, demonstrate the hierarchy of organization in the chemical context of life. Your answer must include the levels of organization, specific examples and the key words: matter, particles, atoms, elements, molecules and compounds.arrow_forwardThere are four sketches below. The first sketch shows a sample of Substance X. The three sketches underneath it show three different changes to the sample. You must decide whether each of these changes is possible. If a change is possible, you must also decide whether it is a physical change or a chemical change.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY