
Concept explainers
(a)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
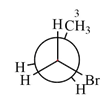
Explanation of Solution
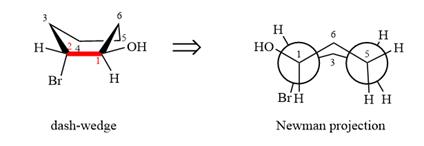
The dash-wedge structure of the molecule is:
The dash-wedge structure shows one hydrogen atom, two carbon atoms on the indicated bond, and the methyl group in one plane. One hydrogen atom on each carbon atom is oriented away from the viewer with bromine, and one hydrogen atom is oriented towards the viewer. For the purpose of determining the positions of the groups in the Newman projection, the carbon bearing the bromine
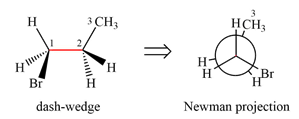
Newman projection represents a molecular structure looking along a particular bond.
(b)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
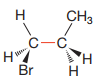
Newman projection of the given structure is:

Explanation of Solution

The dash-wedge structure shows two groups
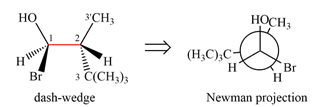
Newman projection represents a molecular structure looking along a particular bond.
(c)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
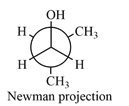
Explanation of Solution
The dash-wedge structure of the molecule is:
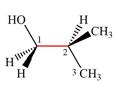
It shows that the carbon on the indicated bond along with
Looking along the indicated bond in
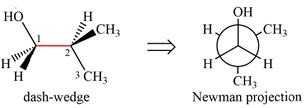
Newman projection represents a molecular structure looking along a particular bond.
(d)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
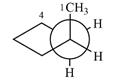
Explanation of Solution
The dash-wedge structure of the molecule is:
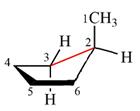
The molecule is substituted cyclopentane. The two groups on each of the carbons C2 and C3 are in axial-equatorial positions. On C3, both are hydrogen atoms. On C2, one is a methyl group in the axial position, and the other is hydrogen in equatorial position.
Looking along the indicated bond in
Therefore, the Newman projection of the given molecule can be drawn as:
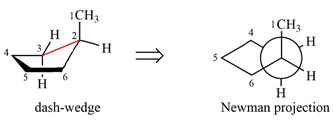
Newman projection represents a molecular structure looking along a particular bond.
(e)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
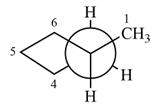
Explanation of Solution
The dash-wedge structure of the molecule is:
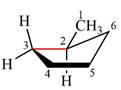
The molecule is substituted cyclopentane. The two groups attached to the ring carbon C2 are methyl in an equatorial up position and hydrogen in an axially down position. The two groups attached to C3 are both hydrogens, one in axial up position and the other in equatorial down position. Looking down the indicated bond in the
The Newman projection of the molecule can therefore be drawn as

Newman projection represents a molecular structure looking along a particular bond.
(f)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
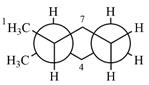
Explanation of Solution
The dash-wedge structure of the molecule is:
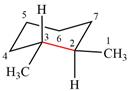
The molecule is substituted cyclohexane in a chair conformation. The methyl groups on the two carbons (C2 and C3) on the indicated bond are both in equatorial positions. The other two groups, both hydrogens, are in axial positions. Looking along the indicated bond in the
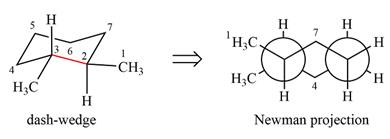
Newman projection represents a molecular structure looking along a particular bond.
(g)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
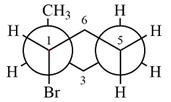
Explanation of Solution
The dash-wedge structure of the molecule is
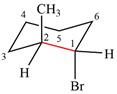
The molecule is substituted cyclohexane in a chair conformation. The two groups attached to C1 are bromine in axial down position and hydrogen in equatorial up position. The two groups attached to C2 are methyl in axial up position and hydrogen in equatorial down position. Looking down the indicated bond, the bromine on the front carbon (C1) and the methyl group on the back carbon (C2), in the Newman projection, will appear straight up and straight down respectively, in staggered positions. The hydrogen on front carbon C1 will appear going to the left, slanted up. The hydrogen on back carbon C2 will appear going left, slanted down. The rest of the ring will appear on right with C6 carbon, on the front. slanted up. C3 carbon, on the back, will appear going to right, slanted down.
The Newman projection of the molecule can then be drawn as
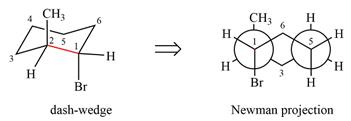
Newman projection represents a molecular structure looking along a particular bond.
(h)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
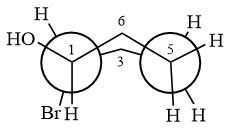
Explanation of Solution
The dash-wedge structure of the molecule is
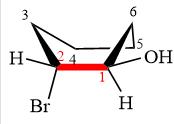
The molecule is substituted cyclohexane in boat conformation. The two groups on C1 are
Looking down the indicated bond, the
Newman projection represents a molecular structure looking along a particular bond.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
