
Fuel oils contain primarily organic compounds and sulfur. The molar composition of the organic fraction of a fuel oil may be represented by the formula
the mass fraction of sulfur in the fuel is
As (kg S/kg fuel); and the percentage excess air, Pn, is defined in terms of the theoretical air required to burn only the carbon and hydrogen in the fuel.
- For a certain high-sulfur No. 6 fuel oil, p = 0.71, 2, SO2, and H2O and expressing the SO2 fraction as ppm (mol SO2/I()6mol dry gas).
- Create a spreadsheet to calculate the mole fractions of the stack gas components on a dry basis for specified values of p, q, r, as, and PXi. The output should appear as follows:
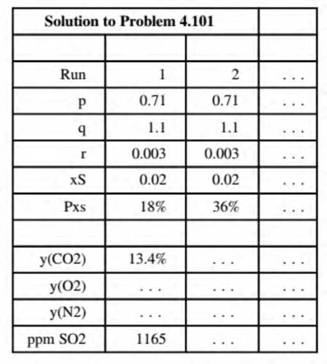
(Rows below the last one shown can be used to calculate intermediate quantities.) Execute enough runs (including the two show n above) to determine the effect on the stack gas composition of each of the five input parameters. Then for the values of p, q, r. and As given in Part (a), find the minimum percentage excess air needed to keep the dry-basis SO2 composition below 700 ppm. (Make this the last run in the output table.)
You should find that for a given fuel oil composition, increasing the percentage excess air decreases the SO2 concentration in the stack gas. Explain w hy this should be the case.
- Someone has proposed using the relationship betw een PX5 and ppm SO2 as the basis of a pollution control strategy. The idea is to determine the minimum acceptable concentration of SO2 in the stack gas, then run with the percentage excess air high enough to achieve this value. Give several reasons w hy this is a poor idea.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Engineering Mechanics: Statics
Mechanics of Materials
Applied Statics and Strength of Materials (6th Edition)
- A fuel composed entirely of methane (CH 4 ) and nitrogen (N 2 ) is burned withexcess air. The flue gases analyze 7.5% CO 2 , 7% O 2 and the remainder, N 2 .Calculate:(a) percentage of excess air(b) analysis of fuel gas(c) analysis of flue gas (wet basis)arrow_forwardQ2: A fuel oil is burned with 65% excess air, and the combustion characteristics of thefuel oil are similar to a paraffin series dodecane C12H26. Determine: (a) the actualair/fuel ratio (A/F) on a mole basis, (b) the actual air/fuel ratio (A/F) on a mass basis,and (c) the actual volumetric (molar) analysis of the products of combustion. State allthe assumptions that you would made before solving this question. Air contains 21%O2 and 79% N2 by mole. Do not solve in tabulated form.arrow_forwardExample 1: Ethane (C2H6) is burned with atmospheric air and the volumetric analysis of the dry products of combustion yields the following: 10% CO2, 1% CO, 3% 02 and 86% N2. Develop the combustion equation and determine (a) the percentage of the excess air (b) the air fuel ratio. (Solve for 100 kmol of dry products).arrow_forward
- 2. The orsat analysis of the products of fuel oil C12H26 = combustion yields: CO₂ = 12.5%, 0₂ = 3.5%, CO = 0.2%, N₂ 83.5%. Determine the actual air fuel ratio (kga/kgf) and the excess air.arrow_forwardHow many of the following are found in 15.0 kmol of xylene (C3H10)? (a) kg C3H10; (b) mol C3H10; (c) Ib-mole C3H10: (d) mol (g-atom) C; (e) mol H; (f) g C; (g) g H; (h) molecules of C3H10.arrow_forwardA fuel containing 80% CH4 and 20% C2H6 is burned with dry air. Sixty percent of the carbon burned goes to CO2, the rest going to CO. Fifty percent excess air is used. Calculate the number of moles of each constituents of the wet flue gas produced by combustion of 100 mole of fuel gas, and also the Orsat analysis of the flue gas.arrow_forward
- 1. In a 100mL sealed flask, 0.1 g of H2 gas reacts with 0.15 g of N2 gas to generate NH3 gas at 25 oC . Note: Consider that i) the initial and final temperatures are 25 oC; ii) all of the limiting reagent is converted to NH3; and iii) the gases behave as ideal. a) Balance the chemical equation :N2(g) + H2(g) ---> NH3(g) b) Calculate the initial pressure inside the flask (before N2 and H2 react). Calculate the initial partial pressures of N2 and H2 (before N2 and H2 react) c) Calculate the final pressure inside the flask (after N2 and H2 react to generate NH3). Calculate the final partial pressures for N2, H2 and NH3 (after N2 and H2 react to generate NH3).arrow_forwardWhen heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction CaCO3(s)→CaO(s) + CO₂(g) What is the mass of calcium carbonate needed to produce 35.0 L of carbon dioxide at STP? Express your answer with the appropriate units. View Available Hint(s) mass of CaCO3 Submit = Part B 0 370.8 HÅ atm Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining ? Butane, C4H10, is a component of natural gas that is used as fuel for cigarette lighters. The balanced equation of the complete combustion of butane is 2C4H10 (9) + 1302 (g)→8CO2 (g) + 10H₂O (1) At 1.00 atm and 23 °C, what is the volume of carbon dioxide formed by the combustion of 2.20 g of butane? Express your answer with the appropriate units.arrow_forward1. A sample of 'raw' natural gas comes out of the ground with, in molar terms, the following composition: 85.0% methane, 3.4% carbon dioxide, 7.7% ethane and 3.9% propane. As part of the purification of methane for distribution, the latter two hydrocarbons are captured for another use. If a million litres of raw gas at STP is processed per day, what would be the combined daily mass of ethane and propane available? a. 1.16 x 105 g b. 2.3 Tonnes 4.46 x 104 g С. d. 4.0 Tonnesarrow_forward
- Long-term space missions require reclamation of the oxygen in the carbon dioxide exhaled by the crew. In one method of reclamation, 1.00 mol of carbon dioxide produces 1.00 mol of oxygen, with 1.00 mol of methane as a by-product. The methane is stored in a tank under pressure and is available to control the attitude of the spacecraft by controlled venting. A single astronaut exhales 1.03 kg of carbon dioxide each day. If the methane generated in the recycling of three astronauts' respiration during one week of flight is stored in an originally empty 140-L tank at −45.0°C, what is the final pressure in the tank?arrow_forwardIf a fuel contains 86% C, 9% H2, 1% S and 4% ash (silica) by mass, determine: (b) (i) the total oxygen needed; (ii) the total air needed, (iii) the stoichiometric air/fuel ratio.arrow_forwardThe oxidation of ethylene to produce ethylene oxide proceeds according to the equation2C 2H 4 +O 2 ! 2C 2H 4OThe feed to a reactor contains 100 kmol C 2H 4 and 100 kmol O 2. If the reaction proceeds to completion, how much of the excess reactant will be left; how much C 2H 4O will be formed; and what is the extent of reaction?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
