
World of Chemistry
7th Edition
ISBN: 9780618562763
Author: Steven S. Zumdahl
Publisher: Houghton Mifflin College Div
expand_more
expand_more
format_list_bulleted
Question
Chapter 4, Problem 36A
Interpretation Introduction
Interpretation:The name and formula of compounds formed by given cation and anion needs to be determined.
Concept Introduction: An ionic compound is composed of cation and anion. Here cation is positively charged and anion carries negative charge.
The cation and anion can be composed of more than one atoms. Such types of ions are called polyatomic ions.
Expert Solution & Answer
Explanation of Solution
Given information:
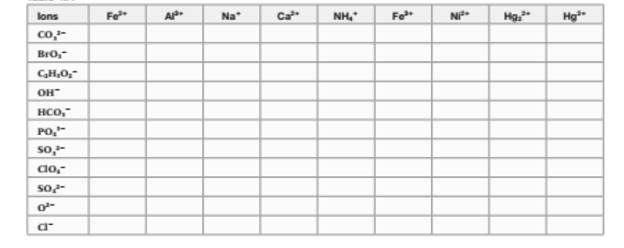
The chemical formula of any compound can be written with criss-cross method in which the valency of both cation and anion are cross multiply to get the simplest whole number of elements in the formula. Thus, the name and formula of compound are listed below;
Ferrous carbonate | Aluminum carbonate | Sodium carbonate | Calcium carbonate | Mercury (I) carbonate | |
iron(II) bromate | Aluminum (III) bromate | Sodium bromate | Calcium bromate | Mercury (II) bromate | |
Iron (II) acetate | Aluminum acetate | Sodium acetate | Calcium acetate | mercury (II) acetate | |
iron (III) hydroxide | Aluminum hydroxide | Sodium hydroxide | Calcium hydroxide | Mercury (II) hydroxide | |
Iron (II) hydrogen carbonate | Aluminum hydrogen carbonate | sodium hydrogen carbonate | calcium hydrogen carbonate | Mercury (II) hydrogen carbonate | |
Iron(II) phosphate | aluminum phosphate | sodium phosphate | calcium phosphate | mercury (II) phosphate | |
iron(II) sulfite | aluminum sulfite | sodium sulfite | calcium sulfite | mercury (II) sulfite | |
iron(II) perchlorate | aluminum perchlorate | sodium perchlorate | calcium perchlorate | Mercury (II) perchlorate | |
iron(II) sulfate | aluminum sulfate | sodium sulfate | calcium sulfate | mercury (II) sulfate | |
iron (II) oxide | aluminum oxide | sodium oxide | calcium oxide | mercury (II) oxide | |
iron (II) chloride | aluminum chloride | sodium chloride | calcium chloride | mercury (II) chloride |
Ammonium carbonate | Ferric carbonate | Nickel carbonate | Mercury (I) carbonate | |
Ammonium bromate | Iron (III) bromate | Nickel (II) bromate | Mercury (I) bromate | |
Ammonium acetate | Iron (III) acetate | Nickel (II) acetate | mercury (I) acetate | |
Ammonium hydroxide | Iron (III) hydroxide | Nickel (II) hydroxide | mercury (I) hydroxide | |
ammonium hydrogen carbonate | iron(III) hydrogen carbonate | nickel (II) hydrogen carbonate | mercury (I) hydrogen carbonate | |
ammonium phosphate | iron(II) phosphate | nickel (II) phosphate | mercury (I) phosphate | |
ammonium sulfite | iron (III) sulfite | Nickel (II) sulfite | Mercury (I) sulfite | |
ammonium perchlorate | iron(III) perchlorate | Nickel(II) perchlorate | Mercury (I) perchlorate | |
ammonium sulfate | iron(III) sulfate | Nickel (II) sulfate | Mercury (I) sulfate | |
Ammonium oxide | iron (III) oxide | Nickel (II) oxide | mercury (I) oxide | |
ammonium chloride | iron (III) chloride | Nickel (II) chloride | mercury (I) chloride |
Conclusion
Criss-cross method is used to find the formula and name of compound by balancing the charges.
Chapter 4 Solutions
World of Chemistry
Ch. 4.1 - Prob. 1RQCh. 4.1 - Prob. 2RQCh. 4.1 - Prob. 3RQCh. 4.1 - Prob. 4RQCh. 4.1 - Prob. 5RQCh. 4.1 - Prob. 6RQCh. 4.2 - Prob. 1RQCh. 4.2 - Prob. 2RQCh. 4.2 - Prob. 3RQCh. 4.2 - Prob. 4RQ
Ch. 4.2 - Prob. 5RQCh. 4.2 - Prob. 6RQCh. 4 - Prob. 1ACh. 4 - Prob. 2ACh. 4 - Prob. 3ACh. 4 - Prob. 4ACh. 4 - Prob. 5ACh. 4 - Prob. 6ACh. 4 - Prob. 7ACh. 4 - Prob. 8ACh. 4 - Prob. 9ACh. 4 - Prob. 10ACh. 4 - Prob. 11ACh. 4 - Prob. 12ACh. 4 - Prob. 13ACh. 4 - Prob. 14ACh. 4 - Prob. 15ACh. 4 - Prob. 16ACh. 4 - Prob. 17ACh. 4 - Prob. 18ACh. 4 - Prob. 19ACh. 4 - Prob. 20ACh. 4 - Prob. 21ACh. 4 - Prob. 22ACh. 4 - Prob. 23ACh. 4 - Prob. 24ACh. 4 - Prob. 25ACh. 4 - Prob. 26ACh. 4 - Prob. 27ACh. 4 - Prob. 28ACh. 4 - Prob. 29ACh. 4 - Prob. 30ACh. 4 - Prob. 31ACh. 4 - Prob. 32ACh. 4 - Prob. 33ACh. 4 - Prob. 34ACh. 4 - Prob. 35ACh. 4 - Prob. 36ACh. 4 - Prob. 37ACh. 4 - Prob. 38ACh. 4 - Prob. 39ACh. 4 - Prob. 40ACh. 4 - Prob. 41ACh. 4 - Prob. 42ACh. 4 - Prob. 43ACh. 4 - Prob. 44ACh. 4 - Prob. 45ACh. 4 - Prob. 46ACh. 4 - Prob. 47ACh. 4 - Prob. 48ACh. 4 - Prob. 49ACh. 4 - Prob. 50ACh. 4 - Prob. 51ACh. 4 - Prob. 1STPCh. 4 - Prob. 2STPCh. 4 - Prob. 3STPCh. 4 - Prob. 4STPCh. 4 - Prob. 5STPCh. 4 - Prob. 6STPCh. 4 - Prob. 7STPCh. 4 - Prob. 8STPCh. 4 - Prob. 9STPCh. 4 - Prob. 10STPCh. 4 - Prob. 11STP
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY