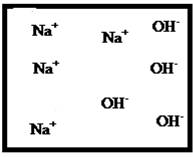
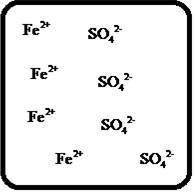
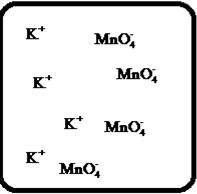
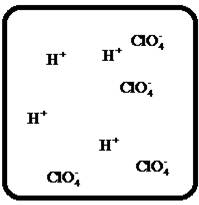
Problem 1RQ: The (aq) designation listed after a solute indicates the process of hydration. Using KBr(aq) and... Problem 2RQ: Characterize strong electrolytes versus weak electrolytes versus nonelectrolytes. Give examples of... Problem 3RQ: Distinguish between the terms slightly soluble and weak. electrolyte. Problem 4RQ: Molarity is a conversion factor relating moles of solute in solution to the volume of the solution.... Problem 5RQ: What is a dilution? What stays constant in a dilution? Explain why the equation M1 V1 = M2 V2 works... Problem 6RQ: When the following beakers are mixed, draw a molecular-level representation of the product mixture... Problem 7RQ: Differentiate between the formula equation, the complete ionic equation, and the net ionic equation.... Problem 8RQ: What is an acid-base reaction? Strong bases are soluble ionic compounds that contain the hydroxide... Problem 9RQ: Define the terms oxidation, reduction, oxidizing agent, and reducing agent. Given a chemical... Problem 10RQ Problem 1ALQ: Assume you have a highly magnified view of a solution of HCl that allows you to see the HCl. Draw... Problem 2ALQ: You have a solution of table salt in water. What happens to the salt concentration (increases,... Problem 3ALQ: You have a sugar solution (solution A) with concentration x. You pour one-fourth of this solution... Problem 4ALQ: You add an aqueous solution of lead nitrate to an aqueous solution of potassium iodide. Draw highly... Problem 5ALQ: Order the following molecules from lowest to highest oxidation state of the nitrogen atom: HNO3,... Problem 6ALQ: Why is it that when something gains electrons, it is said to be reduced? What is being reduced? Problem 7ALQ: Consider separate aqueous solutions of HCl and H2SO4 with the same molar concentrations. You wish to... Problem 8ALQ Problem 9ALQ Problem 10ALQ: The exposed electrodes of a light bulb are placed in a solution of H2SO4 in an electrical circuit... Problem 13Q: Differentiate between what happens when the following are added to water. a. polar solute versus... Problem 14Q: A typical solution used in general chemistry laboratories is 3.0 M HCl. Describe, in detail, the... Problem 15Q Problem 16Q: A student wants to prepare 1.00 L of a 1.00-M solution of NaOH (molar mass = 40.00 g/mol). If solid... Problem 17Q: List the formulas of three soluble bromide salts and three insoluble bromide salts. Do the same... Problem 18Q: When 1.0 mole of solid lead nitrate is added to 2.0 moles of aqueous potassium iodide, a yellow... Problem 19Q: What is an acid and what is a base? An acid-base reaction is sometimes called a proton-transfer... Problem 20Q: A student had 1.00 L of a 1.00-M acid solution. Much to the surprise of the student, it took 2.00 L... Problem 21Q: Differentiate between the following terms. a. species reduced versus the reducing agent b. species... Problem 22Q: How does one balance redox reactions by the oxidation states method? Problem 23E Problem 24E: Match each name below with the following microscopic pictures of that compound in aqueous solution.... Problem 25E: Calcium chloride is a strong electrolyte and is used to salt streets in the winter to melt ice and... Problem 26E: Commercial cold packs and hot packs are available for treating athletic injuries. Both types contain... Problem 27E: Calculate the molarity of each of these solutions. a. A 5.623-g sample of NaHCO3 is dissolved in... Problem 28E: A solution of ethanol (C2H5OH) in water is prepared by dissolving 75.0 mL of ethanol (density = 0.79... Problem 29E: Calculate the concentration of all ions present in each of the following solutions of strong... Problem 30E Problem 31E Problem 32E Problem 33E Problem 34E: If 10. g of AgNO3 is available, what volume of 0.25 M AgNO3 solution can be prepared? Problem 37E: A solution is prepared by dissolving 10.8 g ammonium sulfate in enough water to make 100.0 mL of... Problem 38E: A solution was prepared by mixing 50.00 mL of 0.100 M HNO3 and 100.00 mL of 0.200 M HNO3. Calculate... Problem 39E: Calculate the sodium ion concentration when 70.0 mL of 3.0 M sodium carbonate is added to 30.0 mL of... Problem 40E: Suppose 50.0 mL of 0.250 M CoCl2 solution is added to 25.0 mL of 0.350 M NiCl2 solution. Calculate... Problem 41E Problem 42E: A stock solution containing Mn2+ ions was prepaned by dissolving 1.584 g pure manganese metal in... Problem 43E: On the basis of the general solubility rules given in Table 6-1, predict which of the following... Problem 44E: On the basis of the general solubility rules given in Table 6-1, predict which of the following... Problem 45E: When the following solutions are mixed together, what precipitate (if any) will form? a. FeSO4(aq) +... Problem 46E: When the following solutions are mixed together, what precipitate (if any) will form? a.... Problem 47E: For the reactions in Exercise 47, write the balanced formula equation, complete ionic equation, and... Problem 48E: For the reactions in Exercise 48, write the balanced formula equation, complete ionic equation, and... Problem 49E: Write the balanced formula and net ionic equation for the reaction that occurs when the contents of... Problem 50E: Give an example how each of the following insoluble ionic compounds could be produced using a... Problem 51E: Write net ionic equations for the reaction, if any, that occurs when aqueous solutions of the... Problem 52E: Write net ionic equations for the reaction, if any, that occurs when aqueous solutions of the... Problem 53E: Separate samples of a solution of an unknown soluble ionic compound are treated with KCl, Na2SO4,... Problem 54E: A sample may contain any or all of the following ions: Hg22+, Ba2+, and Mn2+. a. No precipitate... Problem 55E: What mass of Na2CrO4 is required to precipitate all of the silver ions from 75.0 mL of a 0.100-M... Problem 56E: What volume of 0.100 M Na3PO4 is required to precipitate all the lead(II) ions from 150.0 mL of... Problem 57E: What mass of iron(III) hydroxide precipitate can be produced by reacting 75.0 mL of 0.105 M... Problem 60E: What mass of silver chloride can be prepared by the reaction of 100.0 mL of 0.20 M silver nitrate... Problem 61E: A 100.0-mL aliquot of 0.200 M aqueous potassium hydroxide is mixed with 100.0 mL of 0.200 M aqueous... Problem 63E: A 1.42-g sample of a pure compound, with formula M2SO4, was dissolved in water and treated with an... Problem 64E: You are given a 1.50-g mixture of sodium nitrate and sodium chloride. You dissolve this mixture into... Problem 65E: Write the balanced formula, complete ionic, and net ionic equations for each of the following... Problem 66E: Write the balanced formula, complete ionic, and net ionic equations for each of the following... Problem 67E: Write the balanced formula equation for the acid-base reactions that occur when the following are... Problem 68E Problem 69E: What volume of each of the following acids will react completely with 50.00 mL of 0.200 M NaOH? a.... Problem 70E Problem 71E: Hydrochloric acid (75.0 mL of 0.250 M) is added to 225.0 mL of 0.0550 M Ba(OH)2 solution. What is... Problem 72E: A student mixes four reagents together, thinking that the solutions will neutralize each other. The... Problem 73E: A 25.00-mL sample of hydrochloric acid solution requires 24.16 mL of 0.106 M sodium hydroxide for... Problem 74E: A 10.00-mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.5062... Problem 75E: What volume of 0.0200 M calcium hydroxide is required to neutralize 35.00 mL of 0.0500 M nitric... Problem 76E: A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M... Problem 77E: A student titrates an unknown amount of potassium hydrogen phthalate (KHC8H4O4, often abbreviated... Problem 78E: The concentration of a certain sodium hydroxide solution was determined by using the solution to... Problem 79E: Assign oxidation states for all atoms in each of the following compounds. a. KMnO4 b. NiO2 c.... Problem 81E: Assign the oxidation state for nitrogen in each of the following. a. Li3N b. NH3 c. N2H4 d. NO e.N2O... Problem 82E: Assign oxidatioo numbers to all the atoms in each of the following. a. SrCr2O7 b. CuCl2 c. O2 d.... Problem 83E: Specify which of the following are oxidationreduction reactions, and identify the oxidizing agent,... Problem 84E: Specify which of the following equations represent oxidationreduction reactions, and indicate the... Problem 85E: Consider the reaction between sodium metal and fluorine (F2) gas to form sodium fluoride. Using... Problem 86E: Consider the reaction between oxygen (O2) gas and magnesium metal to form magnesium oxide. Using... Problem 87E: Balance each of the following oxidationreduction reactions by using the oxidation states method.... Problem 88E: Balance each of the following oxidationreduction reactions by using the oxidation states method.... Problem 89AE: You wish to prepare 1 L of a 0.02-M potassium iodate solution. You require that the final... Problem 90AE: The figures below are molecular-level representations of four aqueous solutions of the same solute.... Problem 91AE Problem 92AE Problem 93AE: Using the general solubility rules given in Table 6-1. name three reagents that would form... Problem 94AE: Consider a 1.50-g mixture of magnesium nitrate and magnesium chloride. After dissolving this mixture... Problem 95AE: A 1.00-g sample of an alkaline earth metal chloride is treated with excess silver nitrate. All of... Problem 96AE: A mixture contains only NaCl and Al2(SO4)3. A 1.45-g sample of the mixture is dissolved in water and... Problem 98AE: A mixture contains only NaCl and Fe(NO3)3. A 0.456-g sample of the mixture is dissolved in water,... Problem 99AE: A student added 50.0 mL of an NaOH solution to 100.0 mL of 0.400 M HCl. The solution was then... Problem 100AE: Some of the substances commonly used in stomach antacids are MgO, Mg(OH)2, and Al(OH)3. a. Write a... Problem 101AE: Acetylsalicylic acid is the active ingredient in aspirin. It took 35.17 mL of 0.5065 M sodium... Problem 102AE: When hydrochloric acid reacts with magnesium metal, hydrogen gas and aqueous magnesium chloride are... Problem 103AE: A 2.20-g sample of an unknown acid (empirical formula = C3H4O3) is dissolved in 1.0 L of water. A... Problem 104AE: Carminic acid, a naturally occurring red pigment extracted from the cochineal insect, contains only... Problem 105AE: Chlorisondamine chloride (C14H20Cl6N2) is a drug used in the treatment of hypertension. A 1.28-g... Problem 106AE: Saccharin (C7H5NO3S) is sometimes dispensed in tablet form. Ten tablets with a total mass of 0.5894... Problem 107AE: Douglasite is a mineral with the formula 2KC1 FeCl2 2H2O. Calculate the mass percent of douglasite... Problem 108AE: Many oxidationreduction reactions can be balanced by inspection. Try to balance the following... Problem 109AE: The blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with an... Problem 110CWP: Calculate the concentration of all ions present when 0.160 g of MgCl2 is dissolved in 100.0 mL of... Problem 111CWP: A solution is prepared by dissolving 0.6706 g oxalic acid (H2C2O4) in enough water to make 100.0 mL... Problem 112CWP: For the following chemical reactions, determine the precipitate produced when the two reactants... Problem 113CWP: What volume of 0.100 M NaOH is required to precipitate all of the nickel(II) ions from 150.0 mL of a... Problem 114CWP Problem 115CWP: A 450.0-mL sample of a 0.257-M solution of silver nitrate is mixed with 400.0 mL of 0.200 M calcium... Problem 116CWP: The zinc in a 1.343-g sample of a foot powder was precipitated as ZnNH4PO4. Strong heating of the... Problem 117CWP: A 50.00-mL sample of aqueous Ca(OH)2 requires 34.66 mL of a 0.944-M nitric acid for neutralization.... Problem 118CWP: When organic compounds containing sulfur are burned, sulfurdioxide is produced. The amount of SO2... Problem 119CWP: Assign the oxidation state for the element Listed in each of the following compounds: Oxidation... Problem 120CP: A 10.00-g sample consisting of a mixture of sodium chloride and potassium sulfate is dissolved in... Problem 121CP: The units of parts per million (ppm) and parts per billion (ppb) are commonly used by environmental... Problem 122CP: In the spectroscopic analysis of many substances, a series of standard solutions of known... Problem 123CP: In most of its ionic compounds, cobalt is either Co(II) or Co(III). One such compound, containing... Problem 124CP: Polychlorinated biphenyls (PCBs) have been used extensively as dielectric materials in electrical... Problem 125CP: Consider the reaction of 19.0 g of zinc with excess silver nitrite to produce silver metal and zinc... Problem 126CP: A mixture contains only sodium chloride and potassium chloride. A 0.1586-g sample of the mixture was... Problem 127CP Problem 128CP: Zinc and magnesium metal each react with hydrochloric acid according to the following equations:... Problem 129CP: You made 100.0 mL of a lead(II) nitrate solution for lab but forgot to cap it. The next lab session... Problem 130CP: Consider reacting copper(II) sulfate with iron. Two possible reactions can occur, as represented by... Problem 131CP: Consider an experiment in which two burets, Y and Z, are simultaneously draining into a beaker that... Problem 132CP: Complete and balance each acid-base reaction. a. H3PO4(aq) + NaOH(aq) Contains three acidic... Problem 133CP: What volume of 0.0521 M Ba(OH)2 is required to neutralize exactly 14.20 mL of 0.141 M H3PO4?... Problem 134CP: A 10.00-mL sample of sulfuric acid from an automobile battery requires 35.08 mL of 2.12 M sodium... Problem 135CP: A 0.500-L sample of H2SO4 solution was analyzed by taking a 100.0-mL aliquot and adding 50.0 mL of... Problem 136CP: A 6.50-g sample of a diprotic acid requires 137.5 mL of a 0.750 M NaOH solution for complete... Problem 137CP: Citric acid, which can be obtained from lemon juice, has the molecular formula C6H8O7. A 0.250-g... Problem 138CP Problem 139CP: It took 25.06 0.05 mL of a sodium hydroxide solution to titrate a 0.4016-g sample of KHP (see... Problem 140IP Problem 141IP: In a 1-L beaker, 203 mL of 0.307 M ammonium chromate was mixed with 137 mL of 0.269 M chromium(III)... Problem 142IP Problem 143IP: The unknown acid H2X can be neutralized completely by OH according to the following (unbalanced)... Problem 144MP: Three students were asked to find the identity of the metal in a particular sulfate salt. They... Problem 145MP: You have two 500.0-mL aqueous solutions. Solution A is a solution of a metal nitrate that is 8.246%... format_list_bulleted

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




