
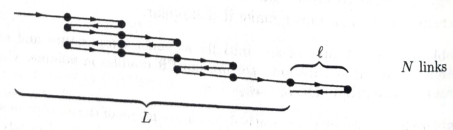
(a) Find an expression for the entropy of this system in term of N and
(b) Write down a formula for L in terms of N and N1,
(c) For a one-dimensional system such as this, the length L is analogous to the volume V of a three-dimensional system. Similarly, the pressure P is replaced by the tension force F. Taking F to be positive when the rubber band is pulling inward, write down and explain the appropriate
(d) Using the thermodynamic Identity, you can flow express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T, N, and
(e) Show that when
(f) Discuss the dependence of the tension force on temperature. If you increase the temperature of a rubber band, does it tend to expand or contract? Does this behavior make sense?
(g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would you expect its temperature to increase or decrease? Explain. Test your prediction with a real rubber band (preferably a fairly heavy one with lots of stretch), using your lips or forehead as a thermometer. (Hint: The entropy you computed in part (a) is not the total entropy of the rubber band. There is additional entropy a8sociated with the vibrational energy of the molecules; this entropy depends on U but is approximately independent of L.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Life in the Universe (4th Edition)
Conceptual Integrated Science
Physics: Principles with Applications
Conceptual Physics (12th Edition)
Applied Physics (11th Edition)
- 1.7 A crystal has a basis of one atom per lattice point and a set of primitive translation vectors (in A): c = 1.5(i + j+ k), a = 3i, where i, j and k are unit vectors in the x, y and z directions of a Cartesian coordinate system. What is the Bravais lattice type of this crystal, and what are the Miller indices of the set of planes most densely populated with atoms? Calculate the volumes of the primitive unit cell and the conventional unit cell. b= 3j.arrow_forwardIn this and following questions, we develop a model for spontaneous emission of a photon by a diatomic molecule AB (a model molecule), which rotates and vibrates. In intermediate calculations, atomic units (a.u.) will be used: unit of mass = the mass of electron, unit of charge is the proton charge e, (e is a positive constant so that the charge of electron is -e). The initial state of the molecule is an excited rotational (1=1) and excited vibrational state (v=1). We consider a molecule with the reduced mass µ = 10,000 a.u. (it is similar to the mass of CO). After emitting a photon, the molecule will go to the 1=0, v=0 state. The first question is about the model potential of the molecule. It is represented by a potential of the form: V(r) = C6 p12 C12 p6 " where r is the distance between A and B in the molecule, C6 and C12 are positive constants (C6 =2 and C₁2-1). This potential has a well meaning that the molecule is bound. The first thing to do is find vibrational states of the…arrow_forwardI'm hoping for a good explanation of how to do this. I'm also wondering why it matters if the configuration is linear or nonlinear? A triatomic molecule can have a linear configuration, as does CO2 (Figure a), or it can be nonlinear, like H2O (Figure b). Suppose the temperature of a gas of triatomic molecules is sufficiently low that vibrational motion is negligible. (a) What is the molar specific heat at constant volume, expressed as a multiple of the universal gas constant (R) if the molecules are linear?Eint/nT = ? (b) What is the molar specific heat at constant volume, expressed as a multiple of the universal gas constant (R) if the molecules are nonlinear?Eint/nT = ? At high temperatures, a triatomic molecule has two modes of vibration, and each contributes 0.5R to the molar specific heat for its kinetic energy and another 0.5R for its potential energy. (c) Identify the high-temperature molar specific heat at constant volume for a triatomic ideal gas of the linear molecules. (Use…arrow_forward
- Consider Ω = αU^(αNV) Use the following steps to find T, U(T), and CV 1. Use quantum mechanics and some combinatorics to find an expression for the multiplicity,Ω , in terms of U, V, N, and any other relevant variables. 2. Take the logarithm to find the entropy, S. 3. Differentiate S with respect to U and take the reciprocal to find the temperature, T, as a function of U and other variables. 4. Solve for U as a function of T (and other variables). 5. Differentiate U(T) to obtain a prediction for the heat capacity (with the other variables held fixed).arrow_forwardConsider two blocks of copper. Block A contains 800 atoms and initially has a total of 20 quanta of energy. Block B contains 200 atoms and initially has 80 quanta of energy. The two blocks are placed in contact with each other, inside an insulated container (so no thermal energy can be exchanged with the surroundings).After waiting for a long time (for example, an hour), which of the following would you expect to be true? A. The entropy of block A is equal to the entropy of block B. B. Approximately 50 quanta of energy are in block A, and approximately 50 quanta of energy are in block B. C. Approximately 80 quanta of energy are in block A, and approximately 20 quanta of energy are in block B. D. The temperature of block A and the temperature of block B are equal.arrow_forwardThree balls (labeled A, B, C) are placed into two different boxes (1 and 2), as in (Figure 1). If all arrangements are equally likely, what is the probability that all three will be in box 1?arrow_forward
- Why is the probability of a system's ground state larger than the probabilities of its excited states for a system in thermal equilibrium with a reservoir? In answering this you may want to consider your calculations in this worksheet. a. Adding energy to the large reservoir increases the total entropy more than adding the same amount of energy to the system. b. Removing all energy from the system increases its entropy the most. c. The probability of the state of the system is maximized when the number of microstates of the system is maximized. d. The probability of the state of the system is maximized when the number of microstates for that state of the system and reservoir is maximized.arrow_forwardA gas consists of three atoms with access to three different quantum states with the same energy. How many different microstates can be formed from these quantum levels for the case of the Fermi-Dirac gas, where the atoms are differentiated but only one atom is allowed in each state.arrow_forwardThe intensities of spectroscopic transitions between the vibrational states of a molecule are proportional to the square of the integral ∫ψv′xψvdx over all space. Use the relations between Hermite polynomials given in Table 7E.1 to show that the only permitted transitions are those for which v′ = v ± 1 and evaluate the integral in these cases.arrow_forward
- Show me the step by step solution. Thank you!arrow_forwardA system containing a single particle in a rigid container at temperature 100 K has a translational partition function of Ztrans = 1.5 × 1012. (a) If the mass of the particle is doubled, what is the new value for ztrans? Report your answer in scientific notation (in Blackboard, 1.23 x 1045 is written 1.23E45).arrow_forwardConsider the throwing of the two dice simultaneously. The sum of the faces of the two dice that are face-up after being thrown (or rolled) can be thought of as representing the state of the dice. What is the total number of possible states of the dice when they are thrown? (Don't forget that each possible state may have a multiplicity greater than one.) What about the probability of finding the dice in the state where their total is 4 after being thrown?arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
