
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 84P
Explain the following:
- a. 1-Hexanol has a higher boiling point than 3-hexanol.
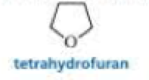
- b. Diethyl ether has very limited solubility in water, but tetrahydrofuran is completely soluble.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The representation of a one-dimensional velocity distribution function for a gas, with increasing temperature the maximum occurs for vi = 0 m/s. Correct?
The representation of a one-dimensional velocity distribution function for a gas, as the temperature increases:a) it becomes more flattenedb) the maximum occurs for vi = 0 m/sExplain it.
The velocity distribution function of gas moleculesa) is used to measure their velocity, since the small size of gas molecules means that it cannot be measured in any other wayb) is only used to describe the velocity of particles if their density is very high.c) describes the probability that a gas particle has a velocity in a given interval of velocities
Chapter 3 Solutions
Organic Chemistry (8th Edition)
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structure of a compound with molecular...Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Prob. 6PCh. 3.1 - Draw the structure for each of the following: a....Ch. 3.1 - Name the following compounds: a. CH3OCH2CH3 b....Ch. 3.2 - Prob. 9PCh. 3.2 - Draw the structure for each of the following: a....Ch. 3.2 - Give each substituent on the nine-carbon chain a...Ch. 3.2 - Prob. 14P
Ch. 3.3 - What is each compounds systematic name?Ch. 3.3 - Prob. 16PCh. 3.3 - Prob. 17PCh. 3.3 - Prob. 18PCh. 3.3 - Prob. 19PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.4 - Prob. 21PCh. 3.5 - a. What is each ethers systematic name? 1....Ch. 3.6 - Give each of the following a systematic name and...Ch. 3.6 - Draw the structures of a homologous series of...Ch. 3.6 - Prob. 25PCh. 3.6 - Prob. 26PCh. 3.7 - Prob. 27PCh. 3.7 - Are the following compounds primary, secondary, or...Ch. 3.7 - Draw condensed and skeletal structures for each of...Ch. 3.7 - For each of the following, give the systematic...Ch. 3.8 - Predict the approximate size of the following bond...Ch. 3.9 - Prob. 32PCh. 3.9 - Prob. 33PCh. 3.9 - Prob. 34PCh. 3.9 - Rank the following compounds from highest boiling...Ch. 3.9 - Rank the compounds in each set from highest...Ch. 3.10 - In which solvent would cyclohexane have the lowest...Ch. 3.10 - Prob. 38PCh. 3.10 - Prob. 39PCh. 3.11 - a. Draw all the staggered and eclipsed conformers...Ch. 3.11 - Prob. 41PCh. 3.11 - Using Newman projections, draw the most stable...Ch. 3.12 - The bond angles in a regular polygon with n sides...Ch. 3.12 - Prob. 44PCh. 3.13 - Draw 1,2,3,4,5,6-hexachlorocydohexane with a. all...Ch. 3.14 - Using the data in Table 3.9, calculate the...Ch. 3.14 - The chair conformer of fluorocyclohexane is 0.25...Ch. 3.15 - Prob. 48PCh. 3.15 - Which has a higher percentage of the...Ch. 3.15 - a. Draw the more stable chair conformer of...Ch. 3.15 - For each of the following disubstituted...Ch. 3.15 - a. Draw Newman projections of the two conformers...Ch. 3.15 - a. Calculate the energy difference between the two...Ch. 3 - a. How many hydrogen does an alkene with 17...Ch. 3 - Draw the structure of octane and isooctaneCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 56PCh. 3 - a. What is each compounds systematic name? b. Draw...Ch. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - Draw a skeletal structure for an alkane that has...Ch. 3 - What is each compounds systematic name? a....Ch. 3 - Which bus a. the higher boiling point:...Ch. 3 - a. Draw Newman projections of the two conformers...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - Prob. 66PCh. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 69PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - For rotation about the C-3C-4 bond of...Ch. 3 - Prob. 72PCh. 3 - What is each compounds systematic name? a. b. c....Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Why are lower molecular weight alcohols more...Ch. 3 - a. Draw a potential energy diagram for rotation...Ch. 3 - For each of the following compound, determine...Ch. 3 - How many ethers have molecular formula C5H12O?...Ch. 3 - Draw the most stable conformer of the following...Ch. 3 - What is each compounds systematic name?Ch. 3 - Calculate the energy difference between the two...Ch. 3 - The most stable from of glucose (blood sugar) is a...Ch. 3 - What is each compound s systematic name?Ch. 3 - Explain the following: a. 1-Hexanol has a higher...Ch. 3 - One of the chair conformers of cis-...Ch. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Name the following compounds:Ch. 3 - Prob. 88PCh. 3 - Using the data obtained in Problem 85, calculate...Ch. 3 - Draw the conformers for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why in the representation of a one-dimensional velocity distribution function for a particular gas, the maximum occurs for vi = 0 m/s.arrow_forwardExplain why the representation of a one-dimensional velocity distribution function for a particular gas becomes flatter as the temperature increases.arrow_forwardDraw a Lewis structure for each of the following molecules and assign charges where appropriate. The order in which the atoms are connected is given in parentheses. a. CIFCIF b. BrCNBrCN 0 c. SOCI2 × (CISCIO) SOC₁₂ (CISCI) You can draw both an octet and a valence shell expanded structure. Considering the following structural information, which is the better one: The measured S-OS-O bond length in SOC12SOCl2 is 1.43 Å. For comparison, that in SO2SO2 is 1.43 Å [Exercise 1-9, part (b)], that in CHзSOHCH3 SOH d. CH3NH2CH3NH2 (methanesulfenic acid) is 1.66 A. e. CH3OCH3 CH3 OCH3 NH2 f. N2H2× (HNNH) N2 H2 (HNNH) g. CH2COCH₂ CO h. HN3× (HNNN) HN3 (HNNN) i. N20 × (NNO) N2O (NNO)arrow_forward
- bre The reaction sequence shown in Scheme 5 demonstrates the synthesis of a substituted benzene derivative Q. wolsd works 2 NH2 NaNO2, HCI (apexe) 13× (1 HNO3, H2SO4 C6H5CIN2 0°C HOTE CHINO₂ N O *O₂H ( PO Q Я Scheme 5 2 bag abouoqmics to sounde odi WEIC (i) Draw the structure of intermediate O. [2 marks] to noitsmot od: tot meinedogm, noit so oft listsb ni zaupaib bas wa (ii) Draw the mechanism for the transformation of aniline N to intermediate O. Spoilage (b) [6 marks] (iii) Identify the reagent X used to convert compound O to the iodinated compound [tom E P. vueimado oilovonsa ni moitos nolisbnolov ayd toes ai tedw nisiqx (iv) Identify the possible structures of compound Q. [2 marks] [2 marks] [shom 2] (v) bus noires goiribbeolovo xnivollot adj to subora sidab Draw the mechanism for the transformation of intermediate P to compound Q. [5 marks] vi (vi) Account for the regiochemical outcome observed in the reaction forming compound Q. [3 marks]arrow_forwardPROBLEM 4 Solved Show how 1-butanol can be converted into the following compounds: a. PROBLEM 5+ b. d. -C= Narrow_forwardWhich alkene is the major product of this dehydration? OH H2SO4 heatarrow_forward
- Please correct answer and don't used hand raiting and don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY