
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 49DSP
Interpretation Introduction
Interpretation:
The orientation of the
Concept introduction:
The basic chair conformation of cyclohexane is shown below:
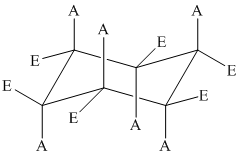
The substituents on alternate carbon atoms, which are on the same side of the ring, have the same orientation – either axial or equatorial.
Two substituents are cis to each other if they are on the same side of the ring.
Two substituents are trans to each other if they are on the opposite side of the ring.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
This molecule (is or is not)
a
reducing sugar.
The sugar molecule is attached to the alcohol at
carbon
(just use a number) in
the steroid structure.
The lactone group is attached to carbon
(just use a number) in the
steroid structure.
There are
(just use a number)
chiral carbons in this molecule.
CH3
HỌ
но.
но,
H
A ÕH
но
ÕH
CH3
• 8H2O
ОН ОН
Xylulose has the following structural formula. To what carbohydrate class does xylulose belong based on the number of carbons and carbonyl functionality?
A) aldotetrose
B) aldopentose
C) ketotetrose
D) ketopentose
E) ketohexose
Explain why they would have different chemical properties.
Chapter 3 Solutions
Organic Chemistry - Standalone book
Ch. 3.1 - Identify the alkanes corresponding to each of the...Ch. 3.1 - Find the conformations in Figure 3.4 in which the...Ch. 3.2 - Sketch a potential energy diagram for rotation...Ch. 3.2 - Acetylcholine is a neurotransmitter in the central...Ch. 3.2 - Prob. 5PCh. 3.5 - The heats of combustion of ethylcyclopropane and...Ch. 3.8 - Prob. 7PCh. 3.10 - The following questions relate to a cyclohexane...Ch. 3.10 - Draw the most stable conformation of...Ch. 3.11 - Prob. 10P
Ch. 3.11 - Prob. 11PCh. 3.12 - Based on what you know about disubstituted...Ch. 3.12 - Write structural formulas for the most stable...Ch. 3.14 - Cubane (C4H8) is the common name of the polycyclic...Ch. 3.14 - Prob. 15PCh. 3.14 - Prob. 16PCh. 3.14 - Prob. 17PCh. 3.14 - Prob. 18PCh. 3.15 - Prob. 19PCh. 3 - Give the IUPAC names of each of the following: (a)...Ch. 3 - Draw Newman projections for the gauche and...Ch. 3 - Identify all atoms that are (a) anti and (b)...Ch. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Oxidation of 4-tert-butylthiane proceeds according...Ch. 3 - The following are representations of two forms of...Ch. 3 - Draw (a) a Newman projection of the most stable...Ch. 3 - Write a structural formula for the most stable...Ch. 3 - Sight down the C-2-C-3 bond, and draw Newman...Ch. 3 - Prob. 34PCh. 3 - Sketch an approximate potential energy diagram for...Ch. 3 - Prob. 36PCh. 3 - Even though the methyl group occupies an...Ch. 3 - Which do you expect to be the more stable...Ch. 3 - Arrange the trimethylcyclohexane isomers shown in...Ch. 3 - Identify the more stable stereoisomer in each of...Ch. 3 - One stereoisomer of 1,1,3,5-tetramethylcyclohexane...Ch. 3 - One of the following two stereoisomers is...Ch. 3 - In each of the following groups of compounds,...Ch. 3 - The heats of combustion of the more and less...Ch. 3 - The measured dipole moment of ClCH2CH2Cl is 1.12D....Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48DSPCh. 3 - Prob. 49DSPCh. 3 - Prob. 50DSPCh. 3 - Prob. 51DSPCh. 3 - Prob. 52DSPCh. 3 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the two monosaccharide units of which trehalose is composedarrow_forwardPalmitoleic acid, a fatty acid with various pharmaceutical applications, is mainly obtained from macadamia nuts. The condensed structural formula for a triacylglycerol containing three palmitoleic acid units is provided below. Which of the following statements is NOT true regarding this triacylglycerol? O || CH,−O−C–(CH,)–CH=CH—(CH2)5–CH3 O || CH−O−C−(CH2)–CH=CH–(CH,)5–CH3 O CH,−O−C−(CH,)–CH=CH–(CH,)5–CH, O Its name is glyceryl tripalmitate or tripalmitin. O It is most likely to be liquid at room temperature. O It is an oil (not a fat). O It contains 3 molecules of the same unsaturated fatty acid.arrow_forwardThe structure of DNA is based on a sugar, deoxyribose, shown below: OH НО Но 2-deoxyribose The cyclic form of deoxyribose contains • stereocentres. The open-chain form of deoxyribose has : stereocentres.arrow_forward
- Lactose contains what number of anomeric carbons and what number of glycosidic bonds: CH2OH ОН CH2OH ОН OH OH OHarrow_forward2. Sucrose is a disaccharide formed from the condensation of glucose and fructose sugars. Draw the structure of sucrose that results from the condensation reaction between the indicated -OH groups. CH2OH 1, CH,OH он 1. OH но CH,OH OH HO H он OHarrow_forwardFill in the empty blanks and spaces in the table. The structure of d-galactose is in the next picture for reference.arrow_forward
- Which of the following is TRUE about amylopectin? - it is a linear polysaccharide composed of D-glucose - accounts for about 20% by weight of starch accounts for about 80% by weight of cellulose - accounts for about 80% by weight of starch - it is a branched polysaccharide of galactose Refer to the molecular formula for the following fatty acids: A. CH3(CH2)10CO2H B. CH3(CH2)12CO2H C. CH3(CH2)14CO2H D. CH3(CH2)16CO2H Е. СН3(СH2)18СО2Н Which of the following statement is TRUE? - Fatty acid C could have the lowest melting point. Fatty acid A could have the highest melting point. - Fatty acid E could have the highest melting point. - Fatty acid D could have the lowest melting point. Refer to the structures of carboxylic acid and derivatives. If pure compound B reacts with pure water, which of the following statements is TRUE? H,N CH3 H,C CH, H,C" CH3 CH A. B D E The product of the reaction is compound A. The product of the reaction is compound C. The product of the reaction is compound D…arrow_forwardHow many chiral carbon atoms are here in the Cholesterol molecule? What is the configuration, R or S, of the carbon atom bonded to the -OH group? What is the configuration, E or Z of the double bond?arrow_forwardKindly answer question i & iiarrow_forward
- Cellulose, the major structural material of wood and plants, consists of a chain of B-D- glucose molecules connected by B-1,4-glycosidic bonds. In comparison, the amylose component of starch consists of a-D-glucose molecules connected by a-1,4-glycosidic bonds. Identify the following structures, denoted A and B, as the structures of cellulose or amylose. CH,OH CH2OH CH2OH CH2OH H. H H H H. H. OH H. H. H. ОН Н H Н но O. Н но H. OH H. ОН H. OH H OH CH2OH CH2OH H. CH2OH H. H. ОН Н ОН Н H CH2OH H H. ОН Н H. H OH H. H. H OH OH H H. H. ОН H. OH O B is the structure of cellulose, while A is the structure of amylose. O A and B both represent the structure of cellulose. O A and B both represent the structure of amylose. O Ais the structure of cellulose, while B is the structure of amylose.arrow_forwardTrue or false 4-bromoaniline could also be named o-bromoaniline. A typical unsaturated fatty acid has a trans double bond in its structure. Trytophan is a hydrophillic, non-polar amino acid. Glucose is a substrate. The substrate for the enzyme succinate dehydrogenase is succinate. If hydrogen bonding between amino acids in the same polypeptide give a coiled shape to the protein we have an example of primary protein structure. A salt bridge would be the type of interaction between two leucines in a tertiary protein structure. Isomerases catalyze the bonding of molecules using ATP energy.arrow_forwardCortisone is a compound that is used as an anti-inflammatory drug to treat 2) diseases like arthritis. Cortisone is a steroid which contains four rings (A, B, C and D), and has the following structure: он "OH Cortisone Provide the data to complete each of the following statements: The total number of chiral carbons (stereocentres) in Cortisone is The maximum number of stereoisomers possible is: The degree of unsaturation (IHD) of Cortisone is The total number of 2° hydrogens is The ring fusion between rings B and C is trans or cis? The absolute configuration of the carbon with * in the structure is The total number of n bonds isarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning