
Concept explainers
Identify the more stable stereoisomer in each of the following pairs, and give the reason for your choice:

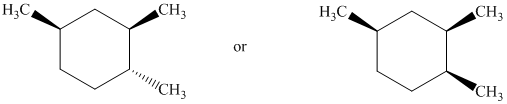
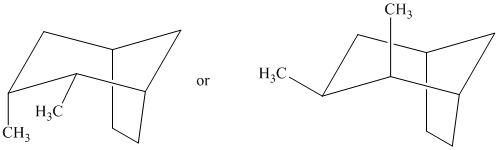
Interpretation:
The most stable stereoisomer in each of the given pairs is to be identified and the reason for it is to be explained.
Concept introduction:
The most stable conformation is the one that has the largest number of substituents in the equatorial orientation.
An axial substituent in the molecule is said to be crowded because of
Crowding causes increase in the potential energy of an isomer, which decreases its stability.
The basic chair conformation of cyclohexane is shown below:
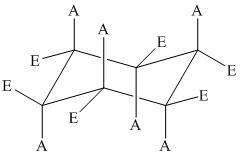
Stereoisomers are isomers having the same constitution but differ in the arrangement of atoms in space. Cis-trans isomers are stereoisomers.
Two substituents are cis to each other if they are on the same side of the ring.
Two substituents are trans to each other if they are on the opposite side of the ring.
Answer to Problem 40P
Solution:
a)
In the cis isomer, the methyl substituent is in the axial orientation while in the trans isomer, the methyl substituent is in the equatorial orientation. The axial methyl group experiences
Thus, the trans isomer (B) is more stable than the cis isomer (A).
b)
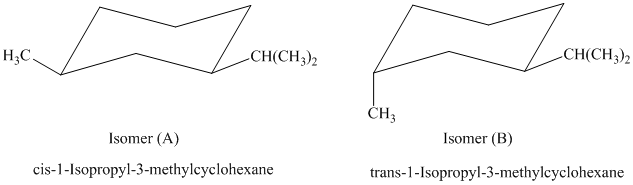
In isomer (A), the methyl substituent is in the equatorial orientation while in isomer (B), the methyl substituent is in the axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
c)
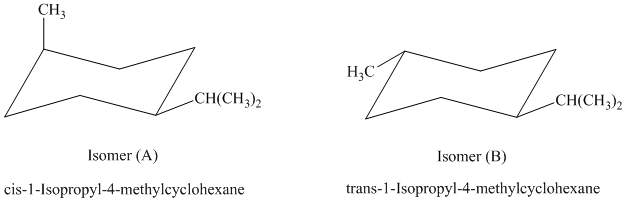
In isomer (A), the methyl substituent is in the axial orientation while in isomer (B), the methyl substituents is in the equatorial orientation. The axial methyl group in isomer (A) experiences
Thus, isomer (B) is more stable than isomer (A).
d)
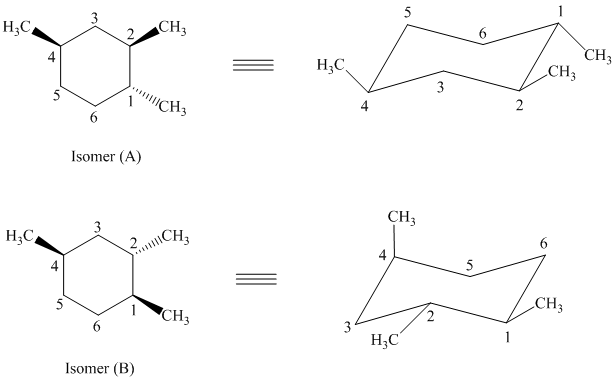
In isomer (A), all three methyl substituents are in an equatorial orientation while in isomer (B), one methyl substituent
Thus, isomer (A) is more stable than isomer (B).
e)
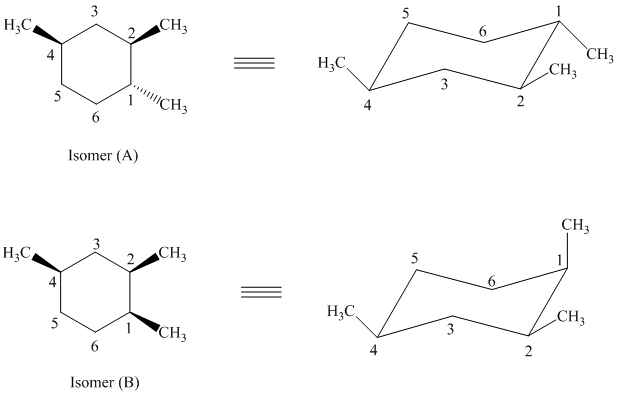
In isomer (A) all three methyl substituents are in an equatorial orientation while in isomer (B), one methyl substituent is in the axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
f)
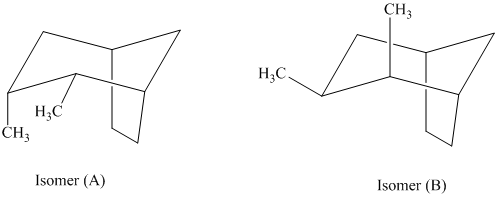
In isomer (A), the two methyl groups are on the same side as the cyclopentane ring and its hydrogen atoms. In isomer (B), the two methyl groups are on the opposite side of the cyclopentane ring and its hydrogen atoms. Isomer (A) is more crowded than isomer (B). Crowding increases the potential energy of the molecule and makes the molecule less stable.
Thus, isomer (B) is more stable than isomer (A).
Explanation of Solution
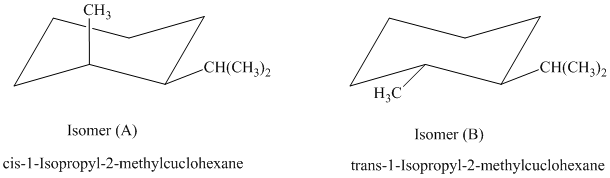
a) The given name of the compound shown below is
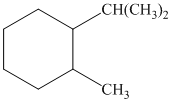
For cis and trans isomers of this compound, two substituents, an isopropyl and methyl groups at
The most stable cis and trans isomers for this compound are shown below:
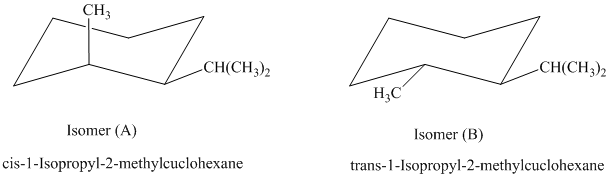
In isomer (A), the methyl substituent is in the axial orientation while in isomer (B), the methyl substituents is in the equatorial orientation. The axial methyl group experiences
Thus, isomer (B) is more stable than isomer (A).
b) The given name of the compound shown below is
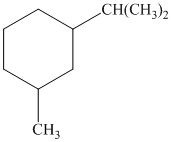
For cis and trans isomers of
The most stable cis and trans isomers for this compound are shown below:
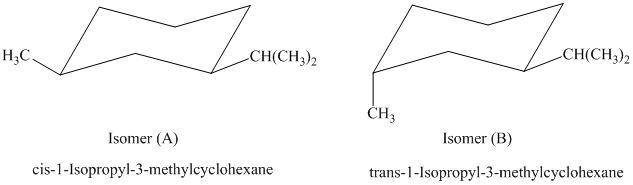
In isomer (A), the methyl substituent is in the equatorial orientation while in isomer (B), the methyl substituent is in the axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
c) The given name of the compound shown below is
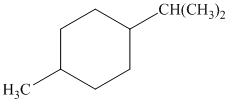
For cis and trans isomers of this compound, two substituents, an isopropyl and methyl groups at
The most stable cis and trans isomers for this compound are shown below:
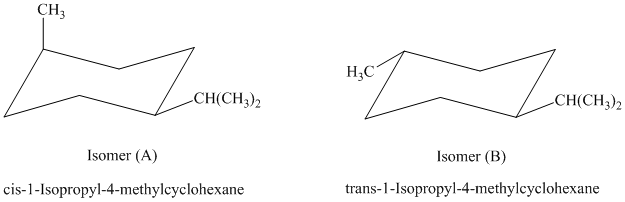
In isomer (A), the methyl substituent is in the axial orientation while in isomer (B), the methyl substituents is in the equatorial orientation. The axial methyl group in isomer (A) experiences
Thus, isomer (B) is more stable than isomer (A).
d) The given structures for two stereoisomers are as follows:
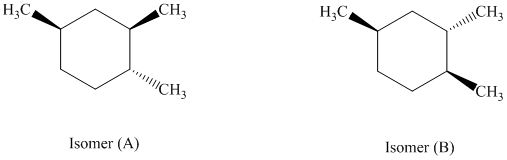
Both of the given isomers have three methyl groups attached to the cyclohexane. Their most stable conformations are shown below:
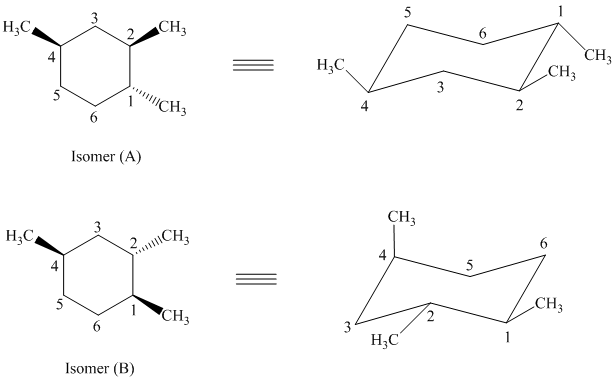
In isomer (A), all three methyl substituents are in an equatorial orientation while in isomer (B), two methyl substituenst at
Thus, isomer (A) is more stable than isomer (B).
e) The given structures for two stereoisomers are as follows:
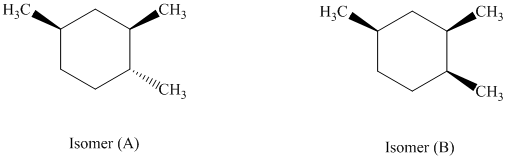
Both of the given isomers have three methyl groups attached to the cyclohexane. Their most stable conformations are shown below:
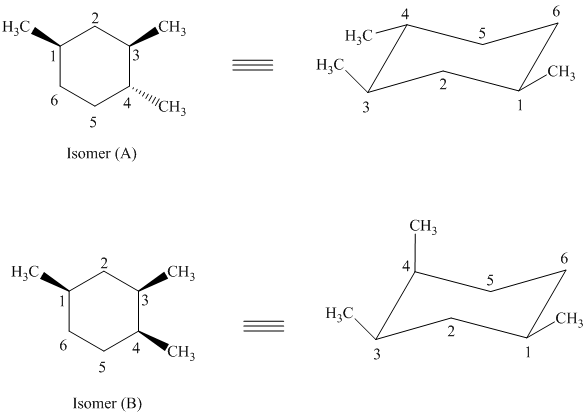
In isomer (A), all three methyl substituents are in an equatorial orientation while in isomer (B), two methyl substituents are in axial orientation. The axial methyl group in isomer (B) experiences
Thus, isomer (A) is more stable than isomer (B).
f) The given structures for two stereoisomers are as follows:
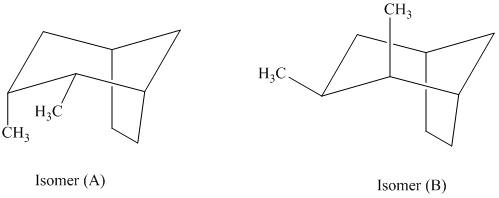
Both of the conformations represent two different cis conformations of the same compound. In isomer (A), the two methyl groups and hydrogen atoms on the cyclopentane ring are on the same side. In isomer (B), the two methyl groups and hydrogen atoms on the cyclopentane rings are on the opposite side. Isomer (A) is more crowded than isomer (B). Crowding increases potential energy of the molecule and makes the molecule less stable.
Thus, isomer (B) is more stable than isomer (A).
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry - Standalone book
- Don't used hand raiting and don't used Ai solutionarrow_forwardDetermine whether the following reaction is an example of a nucleophilic substitution reaction: Br OH HO 2 -- Molecule A Molecule B + Br 义 ollo 18 Is this a nucleophilic substitution reaction? If this is a nucleophilic substitution reaction, answer the remaining questions in this table. Which of the reactants is referred to as the nucleophile in this reaction? Which of the reactants is referred to as the organic substrate in this reaction? Use a ŏ + symbol to label the electrophilic carbon that is attacked during the substitution. Highlight the leaving group on the appropriate reactant. ◇ Yes O No O Molecule A Molecule B Molecule A Molecule B टेarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Show work..don't give Ai generated solutionarrow_forwardPheromone G of the maize stalk borer, chilo partelus, can be synthesized based on the partial scheme shown below. Complete the scheme by identifying the structures of the intermediate compounds A, B, C, D, E, F and pheromone G. Indicate stereochemistry where relevantarrow_forwardQ8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor. одarrow_forward
- Q9: Explain why compound I is protonated on O while compound II is protonated on N. NH2 DD I II NH2arrow_forwardComplete the following reaction by identifying the principle organic product of the reactionarrow_forwardDenote the dipole for the indicated bonds in the following molecules. ✓ H3C CH3 B F-CCl3 Br-Cl H3C —Si(CH3)3 CH3 OH HO HO H HO OH vitamin Carrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

