
Concept explainers
(a)
Interpretation:
The given diagram has to be classified as a mixture, an element or a compound.
The given diagram is,
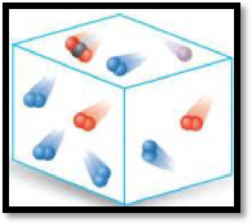
Figure 1
Concept Introduction:
Matter can be classified into two type’s namely pure substance and mixture.
Pure substance: A single component that has a constant composition, irrespective of the
Example: Water, sugar etc.
A pure substance can be classified into an element and a compound.
Element: A pure substance, which cannot be broken down into smaller substances by a
Example: Hydrogen gas, Magnesium ribbon and copper wire etc.
Compound: A pure substance that is formed by combination of two or more elements by chemical process is called as a compound. Example: Sodium chloride is a compound because it is formed from elements sodium and chlorine.
Mixture: A mixture consists of more than one substance and the composition of a mixture is dependent on the sample. The separation of mixture into its components can be done by physical changes.
(b)
Interpretation:
The given diagram has to be classified as a mixture, an element or a compound.
The given diagram is,
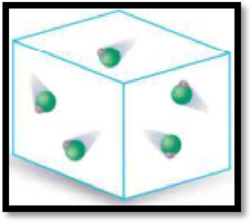
Figure 2
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The given diagram has to be classified as a mixture, an element or a compound.
The given diagram is,
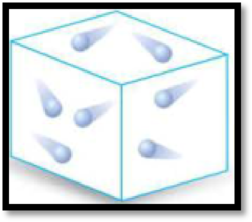
Figure 3
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- In the equation Ni+Cu(NO3)2Ni(NO3)2+Cu, which of the reactants is/are elements, and which of the products is/are compounds?arrow_forwardWhich of the following are elements, and which are compounds? a NaOH; b BaCl2; c He; d Ag; e Fe2O3.arrow_forwardMake molecular-level (microscopic) drawings for each of the following. a. Show the differences between a gaseous mixture that is a homogeneous mixture of two different compounds, and a gaseous mixture that is a homogeneous mixture of a compound and an element. b. Show the differences among a gaseous element, a liquid element, and a solid element.arrow_forward
- Seawater is composed of salt, sand, and water. Is seawatera heterogeneous or homogeneous mixture? Explain.arrow_forwardWhich of the following are compounds, and which are elements? aNa2S bBr2 cPotassium hydroxide dFluorine eCompound or element fCompound or elementarrow_forwardDecide whether each of the following is a physical property or a chemical property of the substance. a Salt substitute, potassium chloride, dissolves in water. b Seashells, calcium carbonate, fizz when immersed in vinegar. c The gas hydrogen sulfide smells like rotten eggs. d Fine steel wool (Fe) can be burned in air. e Pure water freezes at 0C.arrow_forward
- Consider two boxes with the following contents: the first box contains 10 blue paper clips and 10 red paper clips; the second contains the same number of each color of paper clip with the difference that each blue paper clip is interlocked with a red paper clip. Which box has contents that would be an analogy for a mixture, and which box has contents that would be an analogy for a compound?arrow_forwardConsider two boxes with the following contents: the first contains 30 bolts and 30 nuts that fit the bolts; the second contains the same number of nuts and bolts with the difference that each bolt has the nut screwed on it. Which box has contents that would be an analogy for a mixture, and which box has contents that would be an analogy for a compound?arrow_forwardWhich of the following represent physical properties or changes, and which represent chemical properties or changes? You curl your hair with a curling iron. You curl your hair by getting a “permanent wave” at the hair salon. Ice on your sidewalk melts when you put salt on it. A glass of water evaporates overnight when it is left on the bedside table. Your steak chars if the skillet is too hot. Alcohol feels cool when it is spilled on the skin. Alcohol ignites when a flame is brought near it. Baking powder causes biscuits to rise.arrow_forward
- Classify the substances represented by the models in Problem 1-58 as to the number of atoms present per molecule, that is, as diatomic, triatomic, tetraatomic, etc.arrow_forwardClassify each of the following statements as true or false. a. All heterogeneous mixtures must contain three or more substances. b. Pure substances cannot have a variable composition. c. Substances maintain their identity in a heterogeneous mixture but not in a homogeneous mixture. d. Pure substances are seldom encountered in the everyday world.arrow_forwardIced Tea Use iced tea with and without ice cubes as examples to explain homogeneous and heterogeneous mixtures. If you allow all of the ice cubes to melt, what type of mixture remains?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





