
Concept explainers
What amino acid does each abbreviation stand for?
- (a) Phe
- (b) Ser
- (c) Asp
- (d) Gln
- (e) His
- (f) Gly
- (g) Tyr
(a)
Interpretation:
The abbreviation Phe stands for which amino acid has to be given.
Explanation of Solution
The abbreviation Phe stands for the amino acid Phenylalanine.
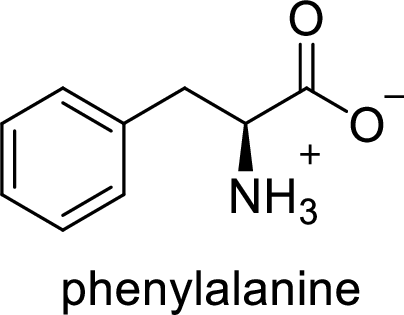
(b)
Interpretation:
The abbreviation Ser stands for which amino acid has to be given.
Explanation of Solution
The abbreviation Ser stands for the amino acid Serine.
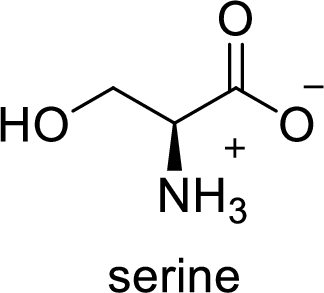
(c)
Interpretation:
The abbreviation Asp stands for which amino acid has to be given.
Explanation of Solution
The abbreviation Asp stands for the amino acid Aspartic acid.
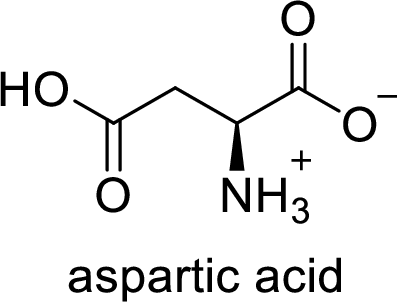
(d)
Interpretation:
The abbreviation Gln stands for which amino acid has to be given.
Explanation of Solution
The abbreviation Gln stands for the amino acid Glutamine.
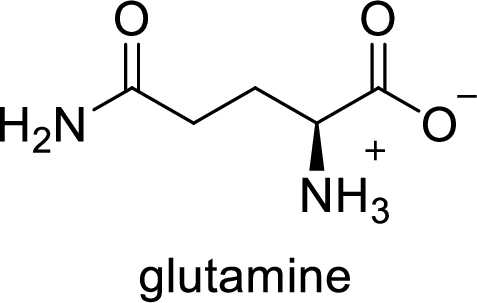
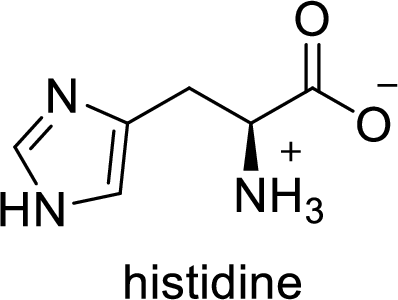
(e)
Interpretation:
The abbreviation His stands for which amino acid has to be given.
Explanation of Solution
The abbreviation His stands for the amino acid Histidine.
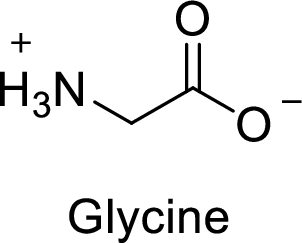
(f)
Interpretation:
The abbreviation Gly stands for which amino acid has to be given.
Explanation of Solution
The abbreviation Gly stands for the amino acid Glycine.
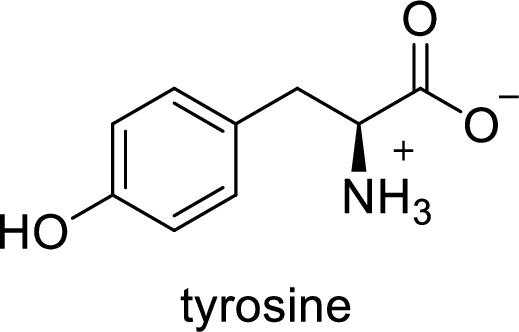
(g)
Interpretation:
The abbreviation Tyr stands for which amino acid has to be given.
Explanation of Solution
The abbreviation Tyr stands for the amino acid Tyrosine.
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
- Electronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forward
- General formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward
- (please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forwardIn the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College DivChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College DivChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





