
Concept explainers
Why spectra of Lyman series only observed when light passes through the hydrogen gas at room temperature.
Answer to Problem 17Q
Solution:
In hydrogen gas transitions involving in the ground state.
Explanation of Solution
Lyman series:
The energy level differences in hydrogen gas follow distinct and recognizable patterns. All the downward transitions that end in the n=1 ground state corresponds to wavelengths in the ultraviolet range of spectrum. This group is known as Lyman series. The series limit for the Lyman series corresponds to n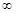 with a wavelength
with a wavelength 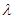 912A°
912A°
At room temperature, nearly all the atoms of hydrogen gas will be in the ground state. When light passes through the gas, an electron in the ground state (n=1)absorbs the photon with wavelength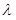 912A°, the electron becomes unbound and it transitions to higher states and creating absorption line.
912A°, the electron becomes unbound and it transitions to higher states and creating absorption line.
Chapter 27 Solutions
Physics: Principles with Applications
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Microbiology: An Introduction
Microbiology: An Introduction
Organic Chemistry (8th Edition)
Applications and Investigations in Earth Science (9th Edition)
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





