
(a)
Interpretation:
The resolution for species B and C from the given data should be determined.
Concept introduction:
The resolution of the column is defined as the separation of two species of the column.
Answer to Problem 26.16QAP
The resolution is
Explanation of Solution
Given:
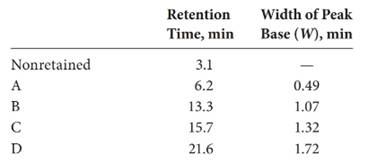
The expression of resolution of the column is:
Here, the retention time of species
Substitute
Thus, the resolution is
(b)
Interpretation:
The selectivity factor for species B and C from the given data should be determined.
Concept introduction:
The resolution of the column is defined as the separation of two species of the column.
Answer to Problem 26.16QAP
The selectivity factor is
Explanation of Solution
The expression of selectivity factor is:
Here, the non-retained retention time is
Substitute
Thus, the selectivity factor is
(c)
Interpretation:
The length of column necessary to separate B and C species with a resolution of
Concept introduction:
The resolution of the column is defined as the separation of two species of the column.
Answer to Problem 26.16QAP
The length of column necessary to separate the two species with a resolution of
Explanation of Solution
The expression of length of the column is:
Here, the number of plates needed to separate the two species is
The expression of relation of the resolution and number of plates is:
Here, the number of plates needed is
Substitute
Substitute
Thus, the length of column necessary to separate the two species with a resolution of
(d)
Interpretation:
The time required to separate B and C species on the column of part c should be determined.
Concept introduction:
The resolution of the column is defined as the separation of two species of the column.
Answer to Problem 26.16QAP
The time required to separate the two species on the column of part c is
Explanation of Solution
The expression of the relation of time required to separate the two species on the column is:
Here, the given resolution is
Substitute
Thus, the time required to separate the two species on the column of part c is
Want to see more full solutions like this?
Chapter 26 Solutions
Principles of Instrumental Analysis
- From the data in Problem 26-14, calculate for species C and D) (a) the resolution. (b) the length of column necessary to separate the two species with a resolution of 2.5.arrow_forwardI need the theoretical molar extinction coefficient of paranitrophenol in mol^-1cm^-1 (using moles not μM). I have to calculate the % difference.At the experimental level it was 7,200 mol^-1cm^-1arrow_forwardExactly 5.00 mL aliquots of a solution containing analyte X were transferred into 50.00-mL volumetric flasks and the pH of the solution is adjusted to 9.0. The following volumes of a standard solution containing 2.00 µg/mL of X were then added into each flask and the mixture was diluted to volume: 0.000, 0.500, 1.00, 1.50 and 2.00 mL. The fluorescence of each of these solutions was measured with a fluorometer, and the following values were obtained: 3.26, 4.80, 6.42, 8.02 and 9.56, respectively. ii. Using relevant functions in Excel, derive a least-squares equation for the data, and use the parameters of this equation to find the concentration of the phenobarbital in the unknown solution.arrow_forward
- A molecular exclusion column has a diameter of 7.8 mm and a length of 30 cm. The solid portion of the particles occupies 20% of the volume, the pores occupy 40%, and the volume between particles occupies 40%. (a) At what volume would totally excluded molecules be expected to emerge? (b) At what volume would the smallest molecules be expected? (c) A mixture of polymers of various molecular masses is eluted between 23 and 27 mL. What does this imply about the retention mechanism for these solutes on the column?arrow_forwardSubstances A and B have retention times of 16.4 and 17.63 min, respectively, on a 30 cm column. An unretained species passes through the column in 1.3 min. The peak width at base for A and B are 1.11 and 1.21 min, respectively. Calculate:arrow_forwardA Dubosq colorimeter consists of a cell of fixed path length and a cell of variable path length. By adjusting the length of the latter until the transmission through the two cells is the same. the concentration of the second solution can be inferred from that of the former. Suppose that a plantdye of concentration 25 μg dm-3 is added to the fixed cell. the length of which is 1.55 em. Then a solution of the same dye. but of unknown concentration. is added to the second cel l. It is found that the same t ransmittance is obtained when the length of the second cel l is adjusted to 1.18 em. What is the concentration of the second solution?arrow_forward
- The retention times of two compounds A and B are 16.40 and 17.63 minutes. A species that is not retained passes through the column in 1.30 minutes. The peak widths (at the base) for A and B are 1.11 and 1.21 minutes, respectively. Calculate:a) The resolution of the columnb) The resolution between compounds A and Barrow_forwardIn color chromatography of plant pigments, what complications would a dried-out column (solvent level is below the top of the silica) introduce to the elution and isolation of pigments?arrow_forward7) A 25.0 ml sample containing Cu gave an instrument reading of 23.6 units (corrected for a blank). When exactly 0.500 ml of 0.0287M Cu(NO3)2 Was added to the solution,the signal increased to 37.9 units. Calculate the molar concentration of Cu" in the sample +2,arrow_forward
- (a) Three components in a mixture have varying distribution constants between the mobile phase and the stationary phase. One of the components has a very high distribution constant. Would this component likely elute first or last and how would you design an experiment or modify the components of the separation to make it perform in the opposite manner? (b) One way to dramatically increase the sensitivity of a quadrupole mass spectrometer is to use a technique called selected ion monitoring (SIM). Explain how this technique works and why it improves sensitivity. Explain what dwell time means. Be sure to draw a diagram and describe how this analysis scheme works. Also, is there a compromise between a wide range of masses scanned and SIM? Explain in easy details.arrow_forwardA peak with a retention time of 10 minutes has a width at half-height of 7.6 s neighboring peak is eluted 15 s later with W½ = 7.5 s. (4) Find out the resolution for these two components. (a) (b) Is this resolution value good for quantitative analysis, why? (c) What difference in retention times is required for an adequate resolution of 1.5?arrow_forward(2) Adenine has a molar absorbance of 4 13.1 M-1cm-1 for 263 nm nucleic acid. What is the molar concentration of an acid solution that shows a permeability of 75% when 2 ml of it is placed in an absorption cell with a thickness of 1 cm?arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

