
Concept explainers
(a)
Interpretation: To identify
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:
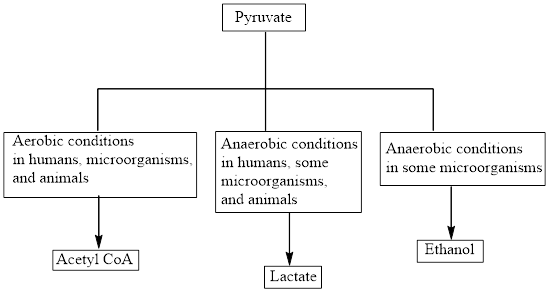
Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
(a)
Answer to Problem 24.45EP
Carbon dioxide
Explanation of Solution
Reason for correct choice:
Under aerobic conditions, pyruvate is converted to
The process of ethanol fermentation takes place in two steps. In step 1, the pyruvate molecule is converted to acetaldehyde by pyruvate decarboxylase enzymes. Carbon dioxide molecule is produced in this step. In step 2, acetaldehyde is reduced to ethanol by alcohol dehydrogenase enzymes. The ethanol fermentation equation is as follows:
Therefore,
Reason for incorrect choice:
The reaction equation for lactate fermentation is as follows:
(b)
Interpretation: To identify NADH is a reactant in which the fate of pyruvate-
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions.
The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Nicotinamide adenine dinucleotide is associated with the
A reactant is defined as the substance that is initially present in the
(b)
Answer to Problem 24.45EP
NADH is encountered as a reactant in the lactate and ethanol production from pyruvate.
Explanation of Solution
Reason for correct choice:
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Ethanol fermentation process occurs in some microorganisms (for example yeast) under the anaerobic conditions. The ethanol fermentation equation is as follows:
Therefore, NADH is encountered as a reactant in the lactate and ethanol production from pyruvate.
Reason for incorrect choice:
The reaction equation for the conversion of pyruvate to
Therefore, NADH is formed along with
(c)
Interpretation: To identify
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Nicotinamide adenine dinucleotide is associated with the redox reactions in metabolism. Its reduced form is NADH and oxidized form is
A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
(c)
Answer to Problem 24.45EP
In the production of
Explanation of Solution
Reason for correct choice:
The reaction equation for the conversion of pyruvate to
Therefore,
Reason for incorrect choice:
The reaction equation for the conversion of pyruvate to lactate is as follows:
Ethanol fermentation process occurs in some microorganisms (for example yeast) under the anaerobic conditions. The ethanol fermentation equation is as follows:
Therefore,
(d)
Interpretation: To identify the end product is a
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Pyruvate
(d)
Answer to Problem 24.45EP
In the absence of oxygen, pyruvate is converted to
Explanation of Solution
Reason for correct choice:
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. This anaerobic reduction is called lactate fermentation. The chemical reaction for the formation of lactate is as follows:

Lactate contains three carbon atoms. Therefore, lactate is a
Reason for incorrect choice:
In the ethanol fermentation process, pyruvate is converted to ethanol and carbon dioxide by enzymes under the anaerobic conditions. The ethanol fermentation equation is as follows:
Ethanol
Pyruvate is converted to
Acetyl group
Want to see more full solutions like this?
Chapter 24 Solutions
General, Organic, and Biological Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





