
Concept explainers
(a)
Interpretation:
Mechanism for the 1st step of the reaction has to be given along with the reason for the direction.
Concept Introduction:
Acid-base reaction:
The species that release proton or accept lone pair of electrons are called acids and the species that accept proton or donate lone pair of electrons are called base.
(b)
Interpretation:
The mechanism for the oxidation step has to be shown.
Concept introduction:
The palladium reagent used as electron transfer reagent to the phenolic oxygen.
(c)
Interpretation:
The mechanism of the respective step has to be given along with the stereochemistry.
Concept introduction:
Diels Alder reaction:
The Diels-Alder reaction is a
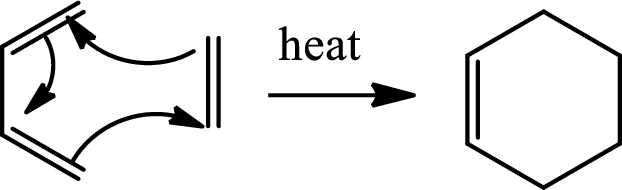
(d)
Interpretation:
Whether the product is racemic or single enantiomer that has to be determined.
Concept introduction:
Racemic mixture:
Racemic mixture is the mixture that has equal amounts of left and right handed enantiomers of the chiral molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- 6. Venlafaxine, an antidepressant, was found to be metabolically unstable. The Medicinal Chemistry team then decided to deuterate the methyl hydrogens as shown below, a strategy which successfully delivered a more stable deuterated analogue (see the transformation below). Using your knowledge of reaction kinetics, explain why such a subtle change resulted in a significantly metabolically stable analogue. OH OH CH3 CD3 I CD H₂CO D₂CO Venlafaxine (antidepressant) Nonadeuterovenlafaxine (antidepressant) -CH3arrow_forwardR-CEN MR-Cool эк-соон Hr write acid catalyzed mechanismarrow_forwardTreatment of acetylene with a suitable base affords lithium acetylide, which was used as a reagent in a partial synthesis of the antitumor natural product (+)-acutiphycin. (Org. Lett. 2014, 16, 1168-1171) Possible bases to consider H-C=C-H Acetylene Step 1 Li :Base T H-O: Li Lithium hydroxide (LiOH) three steps Lithium acetylide Draw the structure of lithium acetylide. Draw Your Solution di. Η Η Η Η | | | | H-C-C-C-C: II HH HH Butyllithium (BuLi) OR CEC- Li OH CH3 -H CH3 Lithium diisopropyl amide (LDA) OMe H3C H H₂C many steps Li HO OH I (+)-Acutiphycin CH3 CH₂ "OHarrow_forward
- Propose a synthesis of the molecule below. You may use benzene, any organic molecule with four or fewer carbons ( with any functional group) and any inorganic reagent you wish.arrow_forwardA) Suggest two possible syntheses for the following target molecule (TM), starting with any aldehyde (start each synthesis with a different aldehyde). It may help to first consider a retrosynthesis of the TM. он CH3. CH-C-CH2CH2CH2CH3 CH3 Hint! Possible aldehyde starting materials: тв для Ph TMarrow_forwardTasks Complete the synthetic sequences by drawing products/substrates/reagents in empty spaces in reactions below. 1. PBr3 2. Mg, dry ether HO 3. CH3CH2C(O)CH3 4. H3O* (acid work-up) 1. MsCI, pyridine 2. CH3CH2SH, acetone ОН 1. LIAIH4 2. H30* 3. conc. H2S04, heat diluted H2SO4 'HO, ОН OH OH 'HO, racemic mixture Page 18 | 18 +arrow_forward
- Following is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. The tributyltin hydride, Bu3SnH, used in the conversion of (H) to (I) reacts via a radical chain reaction; the first step involves a reaction with a radical initiator to form Bu3Sn?. Suggest a mechanism for the rest of the reaction.arrow_forwardFollowing is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. You have not studied the Baeyer-Villiger reaction (D to E). The mechanism involves nucleophilic reaction of the peroxyacid with the carbonyl followed by a rearrangement much like that involved in the hydroboration reaction ). Write a mechanism for this reaction.arrow_forwardFollowing is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. What is the function of the carbon dioxide added to the reaction mixture in Step 2 of the conversion of (E) to (F)? Hint: What happens when carbon dioxide is dissolved in water? Why not just use HCl?arrow_forward
- Following is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. By what type of reaction is (B) converted to (C)?arrow_forwardWhich would be the best way to carry out the following synthesis? OH ... A) (1) t-BUOK, (2) BH3-THF, (3) H,02/NaOH/H,O В NaOH/ H2O (1) t-BuOK, (2) Н,о* (1) NaOCH3, (2) Н,о*arrow_forwardTerpin, prepared commercially by the acid-catalyzed hydration of limonene, is used medicinally as an expectorant for coughs. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism. Arrow-pushing Instructions H3C. H-O-H H-0-H H. H3C CH2 HIO:arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

