
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 24.25P
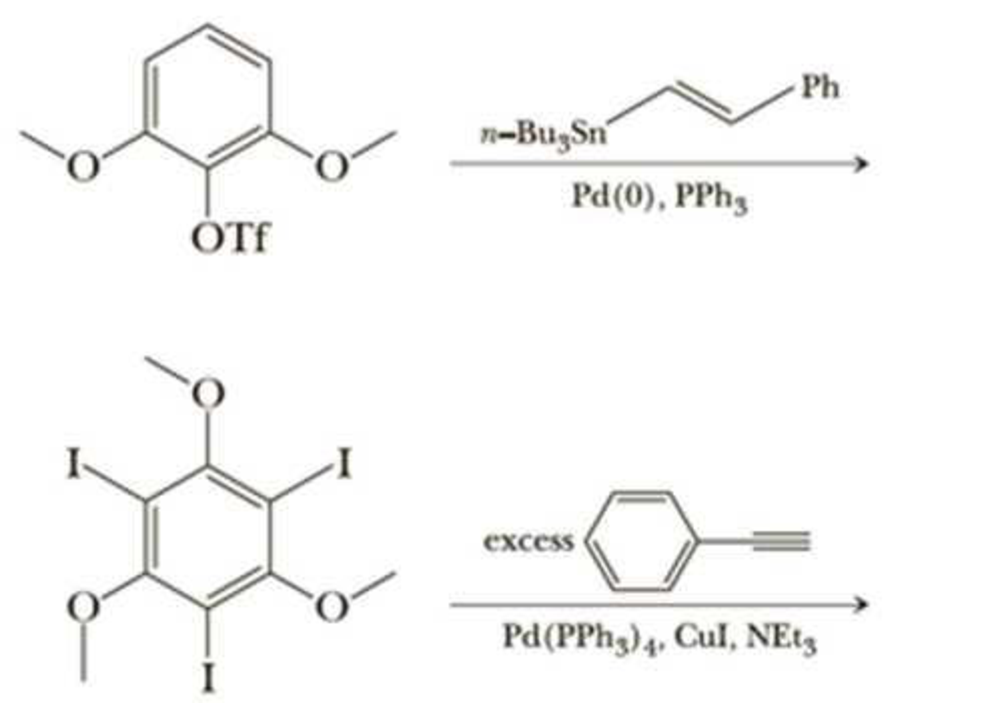
It is typically very difficult to do a substitution reaction on an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
It is typically very difficult to do a substitution reaction on an aromatic ring when the
leaving group is flanked by two other bulky substituents. Moreover, in Section 22.3, we
found that nucleophilic aromatic substitution requires strongly electron-withdrawing
groups on the benzene ring. However, Pd-catalyzed coupling allows entry into such
products. As examples, write the products of the following reactions and state which
coupling reaction is being utilized.
Ph
n-BugSn
Pd(0), PPhg
ÓTf
excess
Pd(PPH3)4, Cul, NE13
The ethylation of benzene is an electrophilic substitution reaction.
Identify the electrophile in the reaction.
Write the equation including reagents and conditions for the ethylation of benzene to produce ethylbenzene.
Hence outline the mechanism for the reaction.
Ethane reacts with chlorine (Cl2) in the presence of ultra-violet (UV) light, to produce 1-chloropropane (CH3CH2Cl) and hydrogen bromide (HCl). As shown in the equation below.CH3CH3+ Cl2→ CH3CH2Cl+ HClDescribe the reaction mechanism of the reaction between ethane and chlorine to produce 1-chloroethane. The description should be detailed and must include the type of bond fission that takes place. You may sketch and insert suitable diagrams to aid your description if you wish.
Chapter 24 Solutions
Organic Chemistry
Ch. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.4 - Prob. 24.3PCh. 24.5 - Show how the following compound can be prepared...Ch. 24.5 - Prob. 24.5PCh. 24.5 - Prob. 24.6PCh. 24.6 - Prob. 24.7PCh. 24 - Prob. 24.8PCh. 24 - Prob. 24.9PCh. 24 - Prob. 24.10P
Ch. 24 - Treatment of cyclohexene with iodobenzene under...Ch. 24 - Prob. 24.12PCh. 24 - Prob. 24.13PCh. 24 - The aryl diene undergoes sequential Heck reactions...Ch. 24 - Heck reactions take place with alkynes as well as...Ch. 24 - Prob. 24.16PCh. 24 - The following transformation involves a series of...Ch. 24 - Show the sequence of Heck reactions by which the...Ch. 24 - Prob. 24.19PCh. 24 - Write the steps that are critical in the following...Ch. 24 - Prob. 24.21PCh. 24 - Prob. 24.22PCh. 24 - Prob. 24.23PCh. 24 - Show how the following compound could be prepared...Ch. 24 - It is typically very difficult to do a...Ch. 24 - The compound eutypine is an antibacterial agent...Ch. 24 - Prob. 24.27PCh. 24 - Prob. 24.28PCh. 24 - Prob. 24.29PCh. 24 - Prob. 24.30PCh. 24 - Prob. 24.31PCh. 24 - Prob. 24.32PCh. 24 - Prob. 24.33PCh. 24 - The following transformation can be accomplished...Ch. 24 - Prob. 24.35PCh. 24 - Prob. 24.36PCh. 24 - Prob. 24.37PCh. 24 - Prob. 24.38PCh. 24 - E. J. Coreys 1964 total synthesis of...Ch. 24 - Prob. 24.40P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Alkynes do not react directly with aqueous acid as do alkenes, but will do so in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the OH group adds to the more highly substituted carbon and the H adds to the less highly substituted carbon. The initial product of the reaction is a vinyl alcohol, also called an enol. The enol immediately rearranges to a more stable ketone via tautomerization. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions -X티 Hö: H-O -CH3 -CH3 H30*arrow_forwardThe oxidation of an aldehyde is commonly carried out using potassium permanganate or potassium dichromate. In fact, the carbon alpha to a benzene ring is very reactive, hence it is possible to oxidize an alkyl benzene compound at the alpha position. Give the equation for the reaction of toluene to benzoic acid using potassium permanganate.arrow_forwardWhy does the addition of bromine to fumaric acid require a high temperature for the reaction while other substrates (generic alkanes, alkenes, and alkynes) react with bromine at room temperature?arrow_forward
- The Friedel-Crafts Alkylation of p-xylene n-propyl bromide can result in an isopropyl as well as n-propyl substitution. A) Write the chemical equation involved in this reaction. B) Which will be the major and minor product? What do you expect will be the ratio of n-propyl to isopropyl substitution for p-xylene?arrow_forwardDetermine whether the reactions occur by elimination, nucleophilic substitution, electrophilic addition, or electrophilic substitution.arrow_forwardTrue or False Considering that two carbon chains have equal number of carbons, but one has Fluorine and the other has Iodine, the one with iodine will have a higher boiling point. Mild oxidation of alkenes results to similar product as that of nucleophilic addition of water to aldehydes.arrow_forward
- Biphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?(c) The heat of hydrogenation for biphenyl is about 418 kJ>mol (100 kcal>mol). Calculate theresonance energy of biphenyl.(d) Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain thedifference in the resonance energies of naphthalene and biphenyl.arrow_forwardOnly one of the chlorine atoms in the molecule, 3,4-dichloronitrobenzene, will undergo nucleophilic substitution. Indicate which position will react and provide the expected product for the given reaction using reaction intermediates (resonance structures).arrow_forwardA student prepares a reaction in which hexane is converted to 1,3-hexadiene. In this reaction, what process does hexane undergo? Please explain in detail.arrow_forward
- The questions are about Aldehydes, Ketones, and Carboxylic Acids -Match the reactions to their respective correct set of reagents and catalystsarrow_forwardProvide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the following reactions.arrow_forwardIn the discussion of poly(ether ether ketone), or PEEK, in Section 26.6a.2, we observed that the addition of a tert-butyl group to the aromatic ring increases the solubility of a PEEK in organic solvents. However, when researchers added a second tert-butyl group, as shown here, they found that the solubility of the PEEK decreased. Explain this observation. A PEEK with two tert-butyl groups per repeating unitarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY