
Concept explainers
Treatment of W with
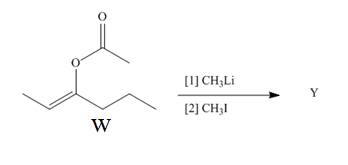
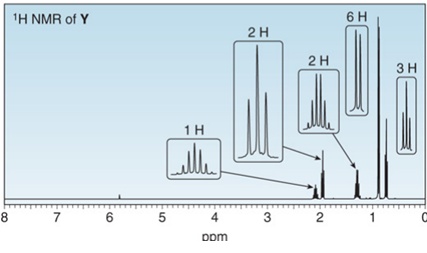
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry
- Show how you would synthesize octan-2-one from each compound. You may use any necessary reagents.(a) heptanalarrow_forward(a) Draw all products formed by treatment of each dibromide (A and B) with one equivalent of NaNH2. (b) Label pairs of diastereomers and constitutional isomers. H Br H Br CH3 CH3 Br H H Br Aarrow_forwardHh.63.arrow_forward
- Compound A undergoes an acid-catalyzed hydrolysis. One of the products (B) that is isolated gives the following 1H NMR spectrum. Identify the compounds A and Carrow_forwardCompound A, MW 72, shows an IR absorption at 1711 cm-1 and a 1H NMR spectrum with peaks at 2.4 8 (2 H, quartet), 2.1 8 (3H, singlet) and 1.1 8 (3 H, triplet). Propose a structure for A. Drawingarrow_forwardConsider carbonyl compounds A–E drawn below. (a) Rank A–E in order of increasing stability. (b) Rank A–E in order of increasing amount of hydrate formed when treated with aqueous acid. (c) Which compound is most reactive in nucleophilic addition?arrow_forward
- Identify the reagents (a–h) needed to carry out each reaction.arrow_forwardTreatment of (CH3)2CHCH(OH)CH2CH3 with TsOH affords two products (M and N) with molecular formula C6H12. The 1H NMR spectra of M and N are given below. Propose structures for M and N, and draw a mechanism to explain their formation.arrow_forwardPropose a structure of compound C (molecular formula C10H12O) consistent with the following data. C is partly responsible for the odor and flavor of raspberries. Compound C: IR absorption at 1717 cm-1arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

