
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 23.66P
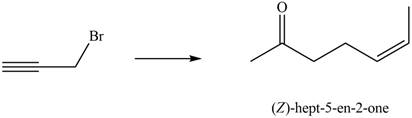
Synthesize (Z)-hept-5-en-2-one from ethyl acetoacetate (CH3COCH2CO2Et) and the given starting material. You may also use any other organic compounds or required inorganic reagents.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
16. The proton NMR spectral information shown in this problem is for a compound with formula
CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec-
tral results, including DEPT-135 and DEPT-90 results, are tabulated:
7
J
Normal Carbon
DEPT-135
DEPT-90
19 ppm
Positive
No peak
122
Positive
Positive
cus
и
124
Positive
Positive
126
Positive
Positive
128
No peak
No peak
4°
129
Positive
Positive
130
Positive
Positive
(144
No peak
No peak
148
No peak
No peak
150
Positive
Positive
してし
3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing
mechanism below, but make sure to draw the product of each proposed step (3 points).
+ En
CN
CN
Show work..don't give Ai generated solution...
Chapter 23 Solutions
Organic Chemistry
Ch. 23 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 23 - Prob. 23.2PCh. 23 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 23 - Prob. 23.4PCh. 23 - Problem 23.5 Which bonds in the following...Ch. 23 - Prob. 23.6PCh. 23 - Prob. 23.7PCh. 23 - Prob. 23.8PCh. 23 - Prob. 23.9PCh. 23 - Prob. 23.10P
Ch. 23 - Problem 23.11 Draw the products of each...Ch. 23 - Problem 23.12 Draw the products of each reaction....Ch. 23 - Prob. 23.13PCh. 23 - Prob. 23.14PCh. 23 - Prob. 23.15PCh. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Problem 23.18 How can pentan-2-one be converted...Ch. 23 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 23 - Problem 23.20 Which of the following compounds...Ch. 23 - Problem 23.21 Draw the products of each...Ch. 23 - Prob. 23.22PCh. 23 - Prob. 23.23PCh. 23 - Prob. 23.24PCh. 23 - Prob. 23.25PCh. 23 - Prob. 23.26PCh. 23 - Prob. 23.27PCh. 23 - Prob. 23.28PCh. 23 - 23.29 Draw enol tautomer(s) for each compound....Ch. 23 - 22.30 The cis ketone A is isomerized to a trans...Ch. 23 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 23 - Prob. 23.32PCh. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - 23.35 Rank the labeled protons in each compound in...Ch. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - 23.38 Acyclovir is an effective antiviral agent...Ch. 23 - 23.39 Explain why forms two different alkylation...Ch. 23 - Prob. 23.40PCh. 23 - 23.41 Acid-catalyzed bromination of pentanone ...Ch. 23 - 23.42 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.43PCh. 23 - Prob. 23.44PCh. 23 - 23.45 Devise a synthesis of valproic acid , a...Ch. 23 - 23.46 Synthesize each compound from diethyl...Ch. 23 - Prob. 23.47PCh. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - 23.50 Draw the organic products formed in each...Ch. 23 - 23.51 Draw the products formed (including...Ch. 23 - Prob. 23.52PCh. 23 - Prob. 23.53PCh. 23 - 23.54 Clopidogrel is the generic name for Plavix,...Ch. 23 - 23.55 What reaction conditions—base, solvent, and...Ch. 23 - Prob. 23.56PCh. 23 - 23.57 Draw a stepwise mechanism showing how two...Ch. 23 - 23.58 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.59PCh. 23 - 23.60 Draw stepwise mechanisms illustrating how...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - 23.63 Synthesize each compound from cyclohexanone...Ch. 23 - Prob. 23.64PCh. 23 - Prob. 23.65PCh. 23 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...Ch. 23 - Prob. 23.67PCh. 23 - 23.68 Capsaicin, the spicy component of hot...Ch. 23 - 23.69 Treatment of W with , followed by , affords...Ch. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72PCh. 23 - Prob. 23.73PCh. 23 - Prob. 23.74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Label the spectrum with spectroscopyarrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? enantiomers H Br H Br (S) CH3 H3C (S) (R) CH3 H3C H Br A Br H C H Br H3C (R) B (R)CH3 H Br H Br H3C (R) (S) CH3 Br H D identicalarrow_forwardLabel the spectrumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY