
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 23.12P
Draw the products of each reaction. Assume excess halogen is present.
a. 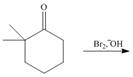 b.
b. 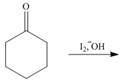 c.
c. 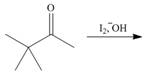
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Correctly name this compound using the IUPAC naming system by sorting the
components into the correct order.
Br
IN
Ν
H
How is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.
Part VI.
(a) calculate the λ max of the compound using woodward - Fieser rules.
(b) what types of electronic transitions are present in the compound?
(c) what are the prominent peaks in the IR spectrum of the compound?
Chapter 23 Solutions
Organic Chemistry
Ch. 23 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 23 - Prob. 23.2PCh. 23 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 23 - Prob. 23.4PCh. 23 - Problem 23.5 Which bonds in the following...Ch. 23 - Prob. 23.6PCh. 23 - Prob. 23.7PCh. 23 - Prob. 23.8PCh. 23 - Prob. 23.9PCh. 23 - Prob. 23.10P
Ch. 23 - Problem 23.11 Draw the products of each...Ch. 23 - Problem 23.12 Draw the products of each reaction....Ch. 23 - Prob. 23.13PCh. 23 - Prob. 23.14PCh. 23 - Prob. 23.15PCh. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Problem 23.18 How can pentan-2-one be converted...Ch. 23 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 23 - Problem 23.20 Which of the following compounds...Ch. 23 - Problem 23.21 Draw the products of each...Ch. 23 - Prob. 23.22PCh. 23 - Prob. 23.23PCh. 23 - Prob. 23.24PCh. 23 - Prob. 23.25PCh. 23 - Prob. 23.26PCh. 23 - Prob. 23.27PCh. 23 - Prob. 23.28PCh. 23 - 23.29 Draw enol tautomer(s) for each compound....Ch. 23 - 22.30 The cis ketone A is isomerized to a trans...Ch. 23 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 23 - Prob. 23.32PCh. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - 23.35 Rank the labeled protons in each compound in...Ch. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - 23.38 Acyclovir is an effective antiviral agent...Ch. 23 - 23.39 Explain why forms two different alkylation...Ch. 23 - Prob. 23.40PCh. 23 - 23.41 Acid-catalyzed bromination of pentanone ...Ch. 23 - 23.42 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.43PCh. 23 - Prob. 23.44PCh. 23 - 23.45 Devise a synthesis of valproic acid , a...Ch. 23 - 23.46 Synthesize each compound from diethyl...Ch. 23 - Prob. 23.47PCh. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - 23.50 Draw the organic products formed in each...Ch. 23 - 23.51 Draw the products formed (including...Ch. 23 - Prob. 23.52PCh. 23 - Prob. 23.53PCh. 23 - 23.54 Clopidogrel is the generic name for Plavix,...Ch. 23 - 23.55 What reaction conditions—base, solvent, and...Ch. 23 - Prob. 23.56PCh. 23 - 23.57 Draw a stepwise mechanism showing how two...Ch. 23 - 23.58 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.59PCh. 23 - 23.60 Draw stepwise mechanisms illustrating how...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - 23.63 Synthesize each compound from cyclohexanone...Ch. 23 - Prob. 23.64PCh. 23 - Prob. 23.65PCh. 23 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...Ch. 23 - Prob. 23.67PCh. 23 - 23.68 Capsaicin, the spicy component of hot...Ch. 23 - 23.69 Treatment of W with , followed by , affords...Ch. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72PCh. 23 - Prob. 23.73PCh. 23 - Prob. 23.74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY