
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 22.65SP
Pentane-2,4-dione (acetylacetone) exists as a tautomeric mixture of 8% keto and 92% enol forms. Draw the stable enol tautomer, and explain its unusual stability.
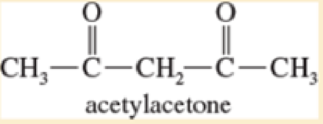
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Describe how 3-methyl-1-phenyl-3-pentanol can be prepared from benzene. You can use any inorganic reagents and solvents, and any organic reagents provided they contain no more than two carbons.
Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+):
CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)
a) Given 7.70 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
b) A chemist ran the reaction and obtained 5.25 g of ethyl butyrate. What was the percent yield?
c) The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.70 g of butanoic acid and excess ethanol?
Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.
Chapter 22 Solutions
Organic Chemistry (9th Edition)
Ch. 22.2A - Prob. 22.2PCh. 22.4 - Without looking back, propose a mechanism for the...Ch. 22.4 - Prob. 22.8PCh. 22.4 - Prob. 22.9PCh. 22.5A - Prob. 22.10PCh. 22.5A - Prob. 22.11PCh. 22.5B - Prob. 22.12PCh. 22.5B - Predict the products of the following reactions....Ch. 22.5B - Which compounds will give positive iodoform tests?...Ch. 22.5C - Propose a mechanism for the acid-catalyzed...
Ch. 22.5C - Acid-catalyzed halogenation is synthetically...Ch. 22.6 - Show the products of the reactions of these...Ch. 22.7A - Prob. 22.18PCh. 22.7A - Prob. 22.19PCh. 22.7A - Prob. 22.20PCh. 22.7B - Prob. 22.21PCh. 22.8 - Prob. 22.22PCh. 22.8 - Prob. 22.24PCh. 22.9 - Prob. 22.25PCh. 22.9 - Prob. 22.26PCh. 22.9 - Prob. 22.27PCh. 22.9 - Prob. 22.28PCh. 22.9 - Prob. 22.29PCh. 22.10 - When cyclodecane-1,6-dione is treated with sodium...Ch. 22.11 - Prob. 22.32PCh. 22.11 - Prob. 22.33PCh. 22.12 - Prob. 22.34PCh. 22.12 - Prob. 22.35PCh. 22.12 - Prob. 22.36PCh. 22.12 - Prob. 22.37PCh. 22.12 - Show what esters would undergo Claisen...Ch. 22.13 - Prob. 22.39PCh. 22.13 - Prob. 22.40PCh. 22.14 - Prob. 22.41PCh. 22.14 - Prob. 22.42PCh. 22.14 - Show how crossed Claisen condensations could be...Ch. 22.14 - Prob. 22.44PCh. 22.14 - Prob. 22.45PCh. 22.15 - Prob. 22.46PCh. 22.16 - Prob. 22.47PCh. 22.16 - Prob. 22.48PCh. 22.17 - Prob. 22.49PCh. 22.17 - Prob. 22.50PCh. 22.17 - Prob. 22.51PCh. 22.18 - Prob. 22.52PCh. 22.18 - Prob. 22.53PCh. 22.18 - Prob. 22.54PCh. 22.18 - Prob. 22.55PCh. 22.18 - Prob. 22.56PCh. 22.19 - Prob. 22.57PCh. 22.19 - Prob. 22.58PCh. 22.19 - Prob. 22.59PCh. 22 - Prob. 22.60SPCh. 22 - 1. Rank the following compounds in order of...Ch. 22 - Prob. 22.62SPCh. 22 - Prob. 22.63SPCh. 22 - Prob. 22.64SPCh. 22 - Pentane-2,4-dione (acetylacetone) exists as a...Ch. 22 - a. Rank these compounds in order of increasing...Ch. 22 - Prob. 22.67SPCh. 22 - Prob. 22.68SPCh. 22 - 22-69 Predict the products of the following...Ch. 22 - Predict the products of these reaction sequences.Ch. 22 - Show how you would accomplish the following...Ch. 22 - Prob. 22.72SPCh. 22 - Prob. 22.73SPCh. 22 - Prob. 22.74SPCh. 22 - The Knoevenagel condensation is a special case of...Ch. 22 - Prob. 22.76SPCh. 22 - Propose mechanisms for the following reactions.Ch. 22 - Prob. 22.78SPCh. 22 - Show how you would accomplish the following...Ch. 22 - Prob. 22.80SPCh. 22 - Propose a mechanism for the following reaction....Ch. 22 - Prob. 22.83SPCh. 22 - Prob. 22.84SPCh. 22 - Prob. 22.85SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.50 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%yield? Express your answer in grams to three significant figures.arrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardDraw the structural formula of the enol formed in each alkyne hydration reaction; then draw the structural formula of the carbonyl compound with which each enol is in equilibriumarrow_forward
- The ketone 2-heptanone has been identified as contributing to the odor of a number of dairy products, including condensed milk and cheddar cheese. Describe the synthesis of 2-heptanone from acetylene and any necessary organic and inorganic reagents.arrow_forwardSynthesize each compound.You may use benzene, acetylene (HC≡CH), ethanol, ethylene oxide, and any inorganic reagents.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forward
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardA 2-bromobutane react with methanol and form a enantiomeric pair of 2-methoxybutane. Draw the structures of the enntiomeric pairs of ethers.arrow_forward1) The carbon-oxygen double bond present in aldehydes and ketones is very polar. What does this mean and how does it arise? 2) The carbon-oxygen double bond is readily attacked by nucleophiles like cyanide ions or ammonia. (i) What do you understand by the term nucleophile? (ii) Which part of the carbon-oxygen double bond is attractive to nucleophiles? 3) Why is there a difference between aldehydes and ketones in their response to oxidizing agents such as potassium dichromate(VI) solution acidified with dilute sulfuric acid?arrow_forward
- Acetylene reacts with sodium amide in the presence of propyl halide produces aldehyde produces ketones It produces 2-pentanearrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. Draw a structural formula for the enol form of the carbonyl compound below.arrow_forwardAn alkene is treated with OsO4 followed by H2O2. When the resulting diol is treated with HIO4, the only product obtained is an unsubstituted cyclic ketone with molecular formula C6H10O. What is the structure of the alkene?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY