
(a)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Grignard reagents are
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
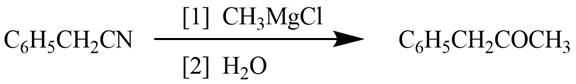
Explanation of Solution
Grignard reagents converts nitriles to corresponding carbonyl groups.
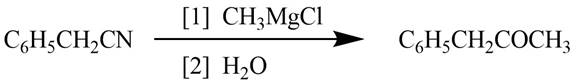
Figure 1
The reagents required to convert phenylacetonitrile
(b)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Grignard reagents are organometallic compounds having the general formula
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
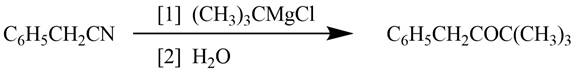
Explanation of Solution
Grignard reagents converts nitriles to corresponding carbonyl groups.
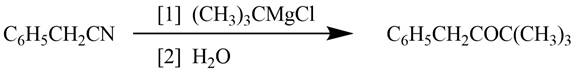
Figure 2
The reagents required to convert phenylacetonitrile
(c)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Nitrile groups get converted to carbonyl group in presence of reducing agents like DIBAL-H.
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
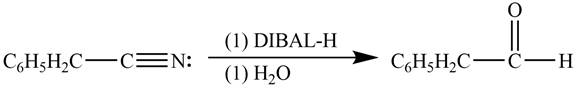
Explanation of Solution
Diisobutylaluminiumhydride(DIBAL-H) is a selective reducing agent. It readily converts nitriles to carbonyl group. DIBAL-H can be used to convert phenylacetonitrile
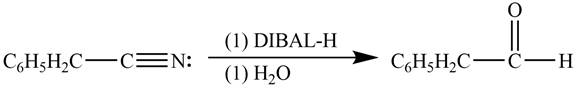
Figure 3
The reagents required to convert phenylacetonitrile
(d)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Hydrolysis of nitriles in acidic medium convert them to the corresponding
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
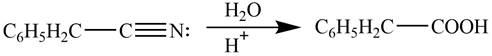
Explanation of Solution
Acidic hydrolysis of phenylacetonitrile
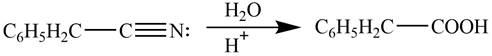
Figure 4
The reagents required to convert phenylacetonitrile
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- Comparing Two Different Methods of Hydration of an Alkene Draw the product formed when CH3CH2CH2CH2CH=CH2 is treated with either (a) H2O, H2SO4; or (b) BH3 followed by H2O2, HO−.arrow_forwardDraw the organic products formed when 2-bromo-3-pentanone (CH,CH,COCHBrCH3) is treated with each reagent: (a) LizCO3, LiBr, DMF; (b) CH;CH2NH2; (c) CH;SH.arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward
- (a) Classify the carbon atoms in each compound as 1°, 2°, 3°, or 4°. (b) Classify the hydrogen atoms in each compound as 1°, 2°, or 3°.arrow_forwardDraw the structure of a compound of molecular formula C 9H 11NO that contains a benzene ring and a: (a) 1 ° amide; (b) 2 ° amide; (c) 3 ° amide.arrow_forward(a) Draw the structures for the eight constitutional isomers of molecular formula C 4H 11N. (b) Give the systematic name for each amine. (c) Identify the chirality center present in one of the amines.arrow_forward
- The following chemical reaction is used to synthesize a flavouring agent that has an aroma similar to bananas. H₂SO4(aq) CH3COOH(1) + CH₂(CH₂)₂OH(1) I || Identify the type of reaction that is represented by this synthesis. Select one: O hydrogenation O esterification O addition O substitution O elimination Identify the functional group and the IUPAC name for each of the three compounds in the reaction below: H₂SO4(aq) I Compound Functional Group IUPAC Name II CH₂COOH(1) + CH₂(CH₂)₂OH(1) I || III CH3COO(CH₂)₂CH₂(1) + H₂O(1) III IV ◆ → ◆ CH3COO(CH₂)₂CH₂(1) + H₂O(1) IV ||| ◆ ◆arrow_forwardWhich structure is a hemiacetal? a) c) OH OCH b) d) CHO OHarrow_forwardIndicate whether each statement is true or false: (a) Fat molecules contain amide bonds. (b) Phosphoplipids can be zwitterions. (c) Phospholipids form bilayers in water in order to have their long hydrophobic tails interact favorably with each other, leaving their polar heads to the aqueous environment.arrow_forward
- 10 Jlgw What are the functional groups present in Aspirin which has the following structural formula aule ulao ut الدرجة من 1 C-OH lio ple P السؤال 0-C-CH3 alcohol , ketone and ether .a O carboxylic acid and ester .b O carboxylic acid , ether and ketone .c C aldehyde and ketone .dOarrow_forwardBile salts are derivative of cholestrol. However, the solubilities of these compounds in water are drastically different; cholestrol is highly hydrophobic, and the bile salts are soluble in digestive juices. Explain the differences.arrow_forwardIdentify the functional groups in two drugs, atenolol and donepezil. Atenololis a β (beta) blocker, a drug used to treat hypertension (high bloodpressure), and donepezil (trade name Aricept) is used to treat mild tomoderate dementia associated with Alzheimer's disease.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



