
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.1, Problem 4P
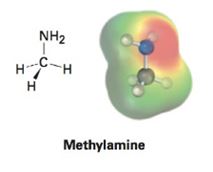
Look at the following electrostatic potential map of methylamine, a substance responsible for the odor of rotting fish, and tell the direction of polarization of the C-N bond:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Give detailed mechanism Solution with explanation needed. don't give Ai generated solution. Don't give Handwritten answer
How will increasing the temperature by ten degrees Celsius impact the rate of reaction?
Select the correct answer below:
the rate will double
the rate will quadruple
the rate will be cut in half
depends on the reaction
Show work. Don't give Ai generated solution. Don't give Handwritten answer
Chapter 2 Solutions
Organic Chemistry
Ch. 2.1 - Prob. 1PCh. 2.1 - Prob. 2PCh. 2.1 - Use the electronegativity values shown in Figure...Ch. 2.1 - Look at the following electrostatic potential map...Ch. 2.2 - Ethylene glycol, HOCH2CH2OH, may look nonpolar...Ch. 2.2 - Make three-dimensional drawings of the following...Ch. 2.3 - Calculate formal charges for the nonhydrogen atoms...Ch. 2.3 - Organic phosphate groups occur commonly in...Ch. 2.6 - Which of the following pairs of structures...Ch. 2.6 - Draw the indicated number of resonance forms for...
Ch. 2.7 - Nitric acid (HNO3) reacts with ammonia (NH3) to...Ch. 2.8 - Prob. 12PCh. 2.8 - Amide ion, H2N-, is a much stronger base than...Ch. 2.9 - Prob. 14PCh. 2.9 - Prob. 15PCh. 2.9 - Prob. 16PCh. 2.11 - Using curved arrows, show how the species in part...Ch. 2.11 - Prob. 18PCh. 2.12 - Of the two vitamins A and C, one is hydrophilic...Ch. 2.SE - Prob. 20VCCh. 2.SE - The following model is a representation of...Ch. 2.SE - cis-l, 2-Dichloroethylene and trans-1,...Ch. 2.SE - The following molecular models are representations...Ch. 2.SE - Predict the product(s) of the acid/base reactions...Ch. 2.SE - Use curved arrows to draw the protonated form of...Ch. 2.SE - Prob. 26MPCh. 2.SE - Double bonds can also act like Lewis bases,...Ch. 2.SE - Prob. 28APCh. 2.SE - Use the electronegativity table given in Figure...Ch. 2.SE - Which of the following molecules has a dipole...Ch. 2.SE - Prob. 31APCh. 2.SE - Phosgene, C12C=O, has a smaller dipole moment than...Ch. 2.SE - Prob. 33APCh. 2.SE - Methanethiol, CH3SH, has a substantial dipole...Ch. 2.SE - Calculate the formal charges on the atoms shown in...Ch. 2.SE - Assign formal charges to the atoms in each of the...Ch. 2.SE - Which of the following pairs of structures...Ch. 2.SE - Prob. 38APCh. 2.SE - 1, 3-Cyclobutadiene is a rectangular molecule with...Ch. 2.SE - Alcohols can act either as weak acids or as weak...Ch. 2.SE - The O-H hydrogen in acetic acid is more acidic...Ch. 2.SE - Draw electron-dot structures for the following...Ch. 2.SE - Write the products of the following acid-base...Ch. 2.SE - Rank the following substances in order of...Ch. 2.SE - Which, if any, of the substances in Problem 2-44...Ch. 2.SE - The ammonium ion (NH4+, pKa = 9.25) has a lower...Ch. 2.SE - Prob. 47APCh. 2.SE - Prob. 48APCh. 2.SE - Calculate Ka values from the following pka’s:...Ch. 2.SE - Calculate pKa values from the following Ka’s:...Ch. 2.SE - What is the pH of a 0.050 M solution of formic...Ch. 2.SE - Prob. 52APCh. 2.SE - Maleic acid has a dipole moment, but the closely...Ch. 2.SE - Assume that you have two unlabeled bottles, one of...Ch. 2.SE - Identify the acids and bases in the following...Ch. 2.SE - Which of the following pairs represent resonance...Ch. 2.SE - Draw as many resonance structures as you can for...Ch. 2.SE - Carbocations, which contain a trivalent,...Ch. 2.SE - We’ll see in the next chapter that organic...Ch. 2.SE - The azide functional group, which occurs in...Ch. 2.SE - Phenol, C6H5OH, is a stronger acid than methanol,...Ch. 2.SE - Thiamin diphosphate (TPP), a derivative of vitamin...Ch. 2.SE - Determine if each compound or ion below has a...Ch. 2.SE - Prob. 64APCh. 2.SE - Prob. 65APCh. 2.SE - Draw the conjugate base for each compound below...Ch. 2.SE - 1, 1, 1-Trichloroethanol is an acid more than 1000...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show work. Don't give Ai generated solutionarrow_forwardExamine the reaction below. Highlight all bonds that have broken in the reactant. Br H H₂C-C- -H 5 H-C-H H-C-H BIE 们 OH H H H₂C-C= H-C-H H-C-Harrow_forwardClassify each of the following as either a substitution, elimination, or addition reaction. CH3 CH3 CH2=CH₂ CH2=C-CH2-OH + Br₂ Br-CH2-C -CH₂-OH Br CH,CH3 CH3-C-CH-CH2-CH3 он ° CH₂ HO C-C-CH3 + NH3 CH₂ он CH3-CH-O-CH3 O-CH3 CH3-C-CH3 + H₂O O-CH3 CH3CH₂ H + H₂O CH3-C=C-CH2-CH3 • CHI Δ Å CH CH3 H+ CH3 C-H + HO-CH3 H" Q-CH₂ CH3-C-CH3 + HO-CH3 OH O substitution O elimination addition substitution O elimination addition O substitution elimination addition O substitution O elimination addition substitution O elimination 00 additionarrow_forward
- Search 5:45 PM Sun Dec 15 Quiz 9 ... ล 25%0 A Done Quiz #9 = Name: Draw the major products of the following. Show Stereochemistry when applicable: 1. OsO4 A 2. NaHSO 3, H 20 Cl₂ ➤ C H2, Pd/C E HBr 1. Hg(OAc) 2, H₂O 2. NaBH 4 Ꭰ KI, H3PO4 F KMnO4, H3O+ KMnO4, H2O G H HBr Br2 J CH2N2 ➤ K CH2I2, Zn(Cu) Cl2, CH3OH C 1. 03 2. Zn, H3O+ HCI 1. BH 3 N M 2. NaOH, H 202 KMnO4, NaOH H₂O P Br2, H2O R 1. BH 3 2. NaOH, H 202 Cl2, CH3CH2OH Tarrow_forwardSelect the stronger base: H-CEN equally basic H H H H-C-N H Select the stronger acid: Select the stronger base: Select the stronger base: H -H equally acidic о equally basic NH equally basic оarrow_forwardClassify each of the following as either a substitution, elimination, or addition reaction. CH3 CH3 CH3-CH-CH2-C-CH3 + Br₂ CH3 CH3 CH3 CH3-C-CH2-C-CH3 + HBr substitution ○ elimination Br CH3 CHI CHO CHA HO CH он Cl CH3-CH2-CH-CH2-CH3 CH₂ DBU H* - CHI CHO CH3 + H2O Ӧ CH3 CH3-CH2-CH=CH-CH3 + HCI OH Pd/C CH3 CH3-CH-CH2-C-CH3 CH3 H C-CH2-CH3 + HO-CH3 addition substitution elimination ○ addition ○ substitution ○ elimination O addition substitution O elimination addition substitution O elimination addition CH3 C-CH3 + H2 CH3 CH3-O-CH-CH2-CH3 Онarrow_forward
- => (8 pts) Use retrosynthetic analysis (that is, use retrosynthetic arrows as was done in class) to suggest a synthesis route for the transformation shown below. Sear bonsarrow_forwardd) 1. Complete the following reactions; all reactions are at room temperature. No heat is involved here. Show Major product only. Indicate the type of mechanism: SN1 or SN2. (1 pt each) a) Br + b) Br e) OH CH3DH + H20 он HCJ Zn Cl₂ OH + HCI 20 C12 + H-Brarrow_forwardWhat is the IUPAC name for the compound shown? LOH IUPAC name: BIU X2 x²arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY