
Concept explainers
Interpretation: Solid, liquid and gas needs to be explained.
Concept Introduction: Matter is something that has mass and volume. Almost everything around us is considered as matter except gas; it is made up of very small particles. All materials are made up of tiny particles, known as atoms.
Answer to Problem 3RQ
Solid, liquids and gases are all made up of molecules, atoms, and ions. The
Explanation of Solution
Almost everything around us is considered as matter except gas; it is made up of very small particles. All materials are made up of tiny particles, known as atoms. Matter exists in the form of solid, liquid and gas. Explanation of solid, liquid and gas with the suitable figure is given below:
Solid: Matter that is composed of tightly packed particles, called Solid. Solids are rigid in comparison to liquids and gases and also, the have a definite shape & volume. Molecules which make up a solid are set in repeating, regular pattern and also, they are held definitely in place (can vibrate within a limited range). Structure of atoms in solid is shown in following figure atom:

Liquid: Matter that is made of more lightly packed particles, called Liquid.The liquid is not so rigid and also, they have a definite volume but no definite shape. Molecule which make up this matter flow easily around one place to another place. They are kept from flying apart by attractive. Structure of atoms in liquid is shown in following figure atom:
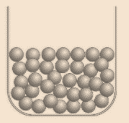
Gas: In gases, atoms & molecules are much more spread out as compared to solids and liquids. Gases are not rigid as compare to liquid and solid. They do not have definite shape and definite volume. Structure of atoms in gas is shown in following figure atom:
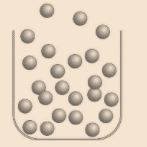
Chapter 2 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





