
Concept explainers
Thioesters
Thioesters have the general formula 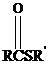 . They resemble their oxygen counterparts
. They resemble their oxygen counterparts 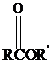
(oxoesters) in structure and reactivity more than other
chlorides, acid anhydrides, and amides. Thioesters can be prepared from thiols by reaction with acyl chlorides or acid anhydrides in much the same way as oxoesters are prepared from
alcohols.
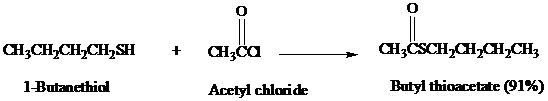
The preparation of thioesters by Fischer esterification is not very effective, however, because
the equilibrium is normally unfavorable. Under conditions in which ethanol is converted to ethyl
benzoate to the extent of
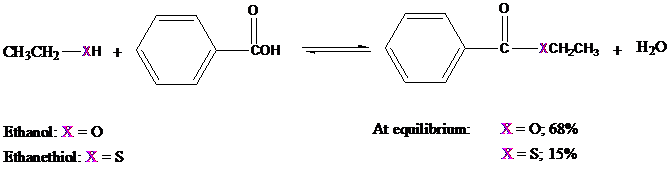
This, and numerous other observations, indicates that
Like chlorine, sulfur is a third-row element and does not act as an electron-pair donor to the carbonyl group as well as oxygen.
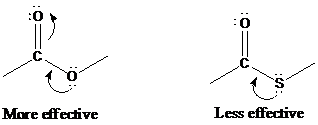
Thioesters and oxoesters are similar in their rates of nucleophilic acyl substitution, except with
amine nucleophiles for which thioesters are much more reactive. Many biological reactions involve nucleophilic acyl substitutions referred to as acyl transfer reactions. The thioester acetyl coenzyme A is an acetyl group donor to alcohols,
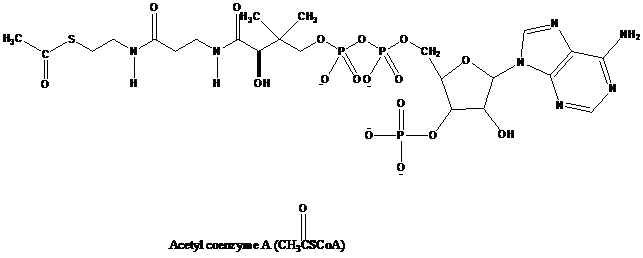 Melatonin, a hormone secreted by the pineal gland that regulates circadian rhythms, including wake–sleep cycles, is biosynthesized by a process in which the first step is an enzyme-catalyzed transfer of the acetyl group from sulfur of acetyl coenzyme A to the
Melatonin, a hormone secreted by the pineal gland that regulates circadian rhythms, including wake–sleep cycles, is biosynthesized by a process in which the first step is an enzyme-catalyzed transfer of the acetyl group from sulfur of acetyl coenzyme A to the

Acetylcholine is a neurotransmitter formed in nerve cells by the enzyme-catalyzed reaction
of choline with acetyl coenzyme A.
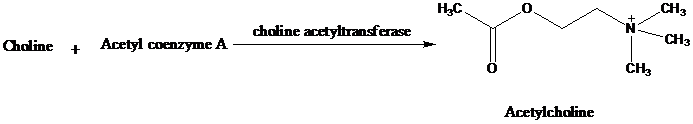
What is the most reasonable structure for choline?
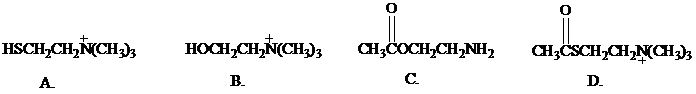
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry - Standalone book
- In the mid-1930s a substance was isolated from a fungus that is a parasite of ryes and other grasses. This alkaloid, lysergic acid, has been of great interest to chemists because of its strange, dramatic action on the human mind. Many derivatives of lysergic acid are known, some with medicinal applications. Perhaps the best known derivative of lysergic acid is the potent hallucinogen lysergic acid diethylamide (LSD): మగవా జి N-H LSD (CH25N;O) Like other alkaloids, LSD is a weak base, with Kp = 7.6 × 107. What is the pH of a 0.94 M solution of LSD? pH =arrow_forwardThe analgesic Tylenol is often taken by people who are allergic to aspirin. Tylenol contains acetaminophen (structure shown) as the active ingredient. Is the structure of acetaminophen similar to the structure of aspirin? In what way? Would acetaminophen give a positive phenol test? What products would be obtained if acetaminophen were hydrolyzed in acidic aqueous solution?arrow_forwardCompare the physical properties of acid derivatives, and explain the unusually highboiling points and melting points of amides. Compare the relative reactivity ofesters, thioesters, amides, nitriles, anhydrides, and acid chloridesarrow_forward
- What reactions and reagents can be used to make phenol from benzene if electrophilic aromatic substitution reactions are excluded and benzene is the only source of carbon?arrow_forwardAlthough p-hydroxybenzoic acid is less acidic than benzoic acid, o-hydroxybenzoic acid is slightly more acidic than benzoic acid. Explain this result.arrow_forwardRänk the carboxylic acids in order of increasing acidity.arrow_forward
- Explain some carboxylic acids in order of decreasing reactivity?arrow_forwardHow to prepare the following compound by the synthesis of malonic acidarrow_forwardMethylparaben is a common preservative used in cosmetics. What carboxylic acid and alcohol are needed to synthesize methylparaben by Fischer esterifi cation?arrow_forward
- Oxalic acid is found in the leaves of rhubarb, primarilyin the form of the calcium salt. Since high levels of oxalicacid are toxic, only rhubarb stalks are used to make strawberry rhubarb pie. What is the IUPAC name of oxalic acid?Write the structure of the calcium salt of oxalic acid.arrow_forwardExplain how the class I carbonyl compound reacts? What will be the product when ethylamine and propyl amine reacts with acetyl chloride? Why only one amide obtained after the reaction of acetyl chloride with a mixture of ethylamine and trimethylamine? Excess amine is required in the reaction of acetyl chloride with amine whereas excess alcohol is not required in the reaction of acetyl chloride and alcohol. Why? List the following ester in order of decreasing reactivities towards hydrolysis with reason: Methyl benzoate, p-nitro methyl benzoate and p-methoxy methyl benzoatearrow_forwardBisphenol A is widely used as a building block in polymer synthesis and is found in the polycarbonate hard plastics of reusable drink containers, DVDs, cell phones, and other consumer goods. Bisphenol A is reported to have estrogenic activity, and its widespread occurrence in our environment is a potential concern. Describe one or two biochemical experiments that could be done to compare the activity of bisphenol A with that of its estradiol, its structural relative.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

