
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20, Problem 49P
Interpretation Introduction
(a)
Interpretation:
Monosaccharide formed after the hydrolysis of given disaccharides needs to be determined.
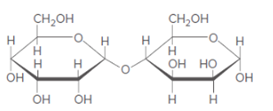
Concept introduction:
When disaccharides are hydrolyzed, a water molecule is added between the glyosidic bond that holds the monosaccharide together and results in the formation of respective monosaccharide.
Interpretation Introduction
(b)
Interpretation:
Monosaccharide formed after the hydrolysis of given disaccharides needs to be determined.
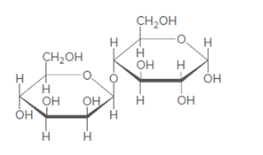
Concept introduction:
When disaccharides are hydrolyzed, a water molecule is added between the glyosidic bond that holds the monosaccharide together and results in the formation of respective monosaccharide.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why in the representation of a one-dimensional velocity distribution function for a particular gas, the maximum occurs for vi = 0 m/s.
Explain why the representation of a one-dimensional velocity distribution function for a particular gas becomes flatter as the temperature increases.
Draw a Lewis structure for each of the following molecules and assign
charges where appropriate. The order in which the atoms are connected
is given in parentheses.
a. CIFCIF
b. BrCNBrCN
0
c. SOCI2 × (CISCIO) SOC₁₂ (CISCI)
You can draw both an octet and a valence
shell expanded structure. Considering the following structural information, which
is the better one: The measured S-OS-O bond length in SOC12SOCl2 is 1.43 Å.
For comparison, that in SO2SO2 is 1.43 Å [Exercise 1-9, part (b)], that in
CHзSOHCH3 SOH
d. CH3NH2CH3NH2
(methanesulfenic acid) is 1.66 A.
e. CH3OCH3 CH3 OCH3
NH2
f. N2H2× (HNNH) N2 H2 (HNNH)
g. CH2COCH₂ CO
h. HN3× (HNNN) HN3 (HNNN)
i. N20 × (NNO) N2O (NNO)
Chapter 20 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 20.1 - Label the hemiacetal carbonthe carbon bonded to...Ch. 20.2 - Prob. 20.1PPCh. 20.2 - Prob. 20.2PCh. 20.2 - Prob. 20.3PCh. 20.2 - Prob. 20.4PCh. 20.2 - Prob. 20.5PCh. 20.2 - Prob. 20.2PPCh. 20.2 - Prob. 20.6PCh. 20.2 - Prob. 20.7PCh. 20.3 - Prob. 20.8P
Ch. 20.3 - Prob. 20.3PPCh. 20.3 - Prob. 20.4PPCh. 20.4 - Prob. 20.5PPCh. 20.4 - Prob. 20.6PPCh. 20.4 - Prob. 20.9PCh. 20.4 - Prob. 20.10PCh. 20.5 - Prob. 20.7PPCh. 20.5 - Prob. 20.8PPCh. 20.5 - Lactose contains both an acetal and a hemiacetal....Ch. 20.5 - Prob. 20.12PCh. 20.5 - Prob. 20.13PCh. 20.5 - Prob. 20.14PCh. 20.5 - Prob. 20.15PCh. 20.6 - Prob. 20.16PCh. 20.6 - Prob. 20.17PCh. 20.7 - Prob. 20.18PCh. 20.7 - Prob. 20.19PCh. 20.8 - Prob. 20.20PCh. 20 - Prob. 21PCh. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Prob. 25PCh. 20 - Prob. 26PCh. 20 - Prob. 27PCh. 20 - Prob. 28PCh. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Prob. 32PCh. 20 - Prob. 33PCh. 20 - Prob. 34PCh. 20 - Prob. 35PCh. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - Prob. 40PCh. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Prob. 43PCh. 20 - Prob. 44PCh. 20 - Prob. 45PCh. 20 - Prob. 46PCh. 20 - What product is formed when each compound is...Ch. 20 - What product is formed when each compound is...Ch. 20 - Prob. 49PCh. 20 - Prob. 50PCh. 20 - Prob. 51PCh. 20 - Prob. 52PCh. 20 - Prob. 53PCh. 20 - Prob. 54PCh. 20 - Prob. 55PCh. 20 - Prob. 56PCh. 20 - Prob. 57PCh. 20 - Prob. 58PCh. 20 - Prob. 59PCh. 20 - Prob. 60PCh. 20 - Prob. 61PCh. 20 - Prob. 62PCh. 20 - Prob. 63PCh. 20 - Prob. 64PCh. 20 - Prob. 65PCh. 20 - Prob. 66PCh. 20 - Prob. 67PCh. 20 - Prob. 68PCh. 20 - Prob. 69PCh. 20 - Prob. 70PCh. 20 - Prob. 71PCh. 20 - Prob. 72PCh. 20 - Prob. 73PCh. 20 - Prob. 74PCh. 20 - Prob. 75PCh. 20 - Prob. 76PCh. 20 - Prob. 77PCh. 20 - Prob. 78PCh. 20 - Prob. 79CPCh. 20 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- bre The reaction sequence shown in Scheme 5 demonstrates the synthesis of a substituted benzene derivative Q. wolsd works 2 NH2 NaNO2, HCI (apexe) 13× (1 HNO3, H2SO4 C6H5CIN2 0°C HOTE CHINO₂ N O *O₂H ( PO Q Я Scheme 5 2 bag abouoqmics to sounde odi WEIC (i) Draw the structure of intermediate O. [2 marks] to noitsmot od: tot meinedogm, noit so oft listsb ni zaupaib bas wa (ii) Draw the mechanism for the transformation of aniline N to intermediate O. Spoilage (b) [6 marks] (iii) Identify the reagent X used to convert compound O to the iodinated compound [tom E P. vueimado oilovonsa ni moitos nolisbnolov ayd toes ai tedw nisiqx (iv) Identify the possible structures of compound Q. [2 marks] [2 marks] [shom 2] (v) bus noires goiribbeolovo xnivollot adj to subora sidab Draw the mechanism for the transformation of intermediate P to compound Q. [5 marks] vi (vi) Account for the regiochemical outcome observed in the reaction forming compound Q. [3 marks]arrow_forwardPROBLEM 4 Solved Show how 1-butanol can be converted into the following compounds: a. PROBLEM 5+ b. d. -C= Narrow_forwardWhich alkene is the major product of this dehydration? OH H2SO4 heatarrow_forward
- Please correct answer and don't used hand raiting and don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forward
- Quantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forwardQuantum mechanics. In translational motion, the summation is replaced by an integral when evaluating the partition function. This is correct becausea) the spacing of the translational energy levels is very small compared to the product kTb) the spacing of the translational energy levels is comparable to the product kTc) the spacing of the translational energy levels is very large compared to the product kTarrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning