
Concept explainers
QUANTITATIVE Bond Energies. A single covalent bond has a bond energy of approximately 90 kcal/mol, and a typical hydrogen bond has a bond energy of about 5 kcal/mol. Although weak, hydrogen bonds can be a major structural force when present in large numbers as in DNA. In double-stranded DNA, each AT base pair is held together by two hydrogen bonds, and each GC base pair is held together by three hydrogen bonds.
(a) What is the total bond energy in a propane molecule (see Figure 2-4)? The C—C bond energy is 83 kcal/mol, and the C—H bond energy is 99 kcal/mol.
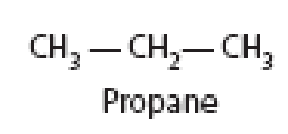
(b) In a short 15 base-pair molecule of DNA having 60% GC pairs and 40% AT pairs, what is the total bond energy of all the hydrogen bonds? How does this compare to the bond energy of a carbon-carbon bond?
(c) In a typical gene consisting of 1000 base pairs with the same relative GC versus AT content, what is the total bond energy of all the hydrogen bonds? How does this compare to the bond energy of a carbon-carbon bond?
Trending nowThis is a popular solution!

Chapter 2 Solutions
Becker's World of the Cell (9th Edition)
- 7 Protein structure.Circle one of the three amino acid sequences that is most likely to form a stable a-helix? RASKTARQ DASKTAEQ KPGKPAGQ In one sentence (that can be accompanied by a small picture) explain why?arrow_forwardSedimenting spheres. What is the dependence of the sedimentation coefficient s of a spherical protein on its mass?How much more rapidly does an 80 -kDa protein sedimentthan does a 40-kDa protein?arrow_forwardNeed help. Which one of the following statements is FALSE? Group of answer choices A.Beta-pleated sheets are part of the secondary structure of proteins B.The nitrogenous bases of DNA are located on the inside because they are hydrophobic in character C.The peptide bond is formed by dehydration synthesis D.Alpha helices are stabilized by attraction between the amino acid R groups E.The peptide bond is rigid and planar and has partial double bond characterarrow_forward
- Alpha-helix of proteins, its characteristics. Draw a diagram of the arrangement of amino acids in the alpha helix. Examples of proteins formed mainly by this structure.arrow_forwardIonization State of Histidine.Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge on the functional group is determined by the relationship between its pKa and the pH of the solution. This relationship is described by the Henderson-Hasselbalch equation. 1.Histidine has three ionizable functional groups. Write the equilibrium equations for its three ion-izationsand assign the proper pKa for each ionization. Draw the structure of histidine in each ionization state.What is the net charge on the histidine molecule in each ionization state? 2.Which structure drawn in (1) corresponds to theionization state of histidine at pH 1, 4, 8, and12?Note that the ionization state can be approximated by treating each ionizable group independently. 3.What is the net charge of histidine at pH 1, 4, 8, and 12? For each pH, will histidine migrate to-ward the anode (+) or cathode (-) when placed in an electric field?arrow_forwardPeptide bond properties. How these properties determine the three-dimensional structure of the protein.arrow_forward
- On the trail of carbons. Tissue culture cells were incubated with glutamine labeled with 15NN in the amide group. Subsequently, IMP was isolated and found to contain some 15N.N. Which atoms in IMP were labeled?arrow_forwardProteins are quite stable. The lifetime of a peptide bond in aqueous solution is nearly 1000 years. However, the free energy of hydrolysis of proteins is negative and quite large. How can you account for the stability of the peptide bond in light of the fact that hydrolysis releases much energy?arrow_forwardBeta folding. Parallel and antiparallel beta folding sheets (be able to draw). Beta loops. Examples of proteins formed mainly by this structure.arrow_forward
- DNA True or false A long chain polymer in which the internucleotide linkages are of the diester type between C-3’ and C-5’ Hydrolyzed by weak alkali (pH 9 to 100°C) Different from RNA since in the latter the internucleotide linkages are between C-2’ and C-5 Usually present in tissues as a nucleoprotein and cannot be separated from its protein componentarrow_forwardOnly galactose need. Solve like samplearrow_forwardPeptide mass determination. You have isolated a proteinfrom the bacterium E. coli and seek to confirm its identityby trypsin digestion and mass spectrometry. Determinationof the masses of several peptide fragments has enabled youto deduce the identity of the protein. However, there is adiscrepancy with one of the peptide fragments, whichyou believe should have the sequence MLNSFK and an(M 1 H)1 value of 739.38. In your experiments, yourepeat edly obtain an (M 1 H)1 value of 767.38. What isthe cause of this discrepancy and what does it tell youabout the region of the protein from which this peptide isderived?arrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





