
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.24P
Which proton in each of the following drugs is most acidic? THC is the active component in marijuana, and ketoprofen is an anti-inflammatory agent.
a.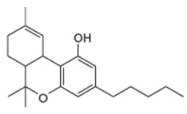 b.
b.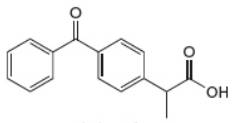
THC ketoprofen
tetrahydrocannabinol
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used Ai solution
this is an organic chemistry question please answer accordindly!!
please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!!
im reposting this kindly solve all parts and draw it not just word explanations!!
Please correct answer and don't used hand raiting
Chapter 2 Solutions
Organic Chemistry
Ch. 2 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2 - a. Draw the conjugate acid of each base:...Ch. 2 - Label the acid and base, and the conjugate acid...Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Which compound in each pair is the stronger acid?...Ch. 2 - Use a calculator when necessary to answer the...Ch. 2 - Rank the conjugate bases of each of group of acids...Ch. 2 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2 - Estimate the pKa of each of the indicated bonds.
Ch. 2 - Draw the products of each reaction and determine...Ch. 2 - Prob. 2.12PCh. 2 - Without reference to a pKa table, decide which...Ch. 2 - which compound in each pair of isomers is the...Ch. 2 - Prob. 2.15PCh. 2 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2 - whichcompound in each pair is the stronger acid? a...Ch. 2 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2 - Explain the apparent paradox. HBr is a stronger...Ch. 2 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2 - Acetonitrile (CH3CN) has a pKa of 25, making it...Ch. 2 - For each pair of compounds: [1] Which indicated H...Ch. 2 - Rank the compounds in each group in order of...Ch. 2 - Which proton in each of the following drugs is...Ch. 2 - Prob. 2.25PCh. 2 - Problem 2.29
Compounds like amphetamine that...Ch. 2 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2 - Which species are Lewis acids?
a. b. c. d.
Ch. 2 - For each reaction, label the Lewis acid and base....Ch. 2 - Prob. 2.30PCh. 2 - Prob. 2.31PCh. 2 - Prob. 2.32PCh. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - Prob. 2.35PCh. 2 - Prob. 2.36PCh. 2 - a Draw the conjugate acid of ethylene, CH2 = CH2....Ch. 2 - 2.40 Draw the products formed from the acid-base...Ch. 2 - Draw the products formed from the acid-base...Ch. 2 - Prob. 2.40PCh. 2 - Draw the product of acid-base reaction. a. c. b....Ch. 2 - Prob. 2.42PCh. 2 - Prob. 2.43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Which compounds can be deprotonated by OH, so that...Ch. 2 - Draw the product of each reaction. Use the pKa...Ch. 2 - Rank the following compounds in order of...Ch. 2 - Rank the following ions in order of increasing...Ch. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - The pKa of three CH bonds is given below. a. For...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - 2.56 Draw the structure of a constitutional isomer...Ch. 2 - 2.57 Many drugs are Bronsted-Lowry acids or...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - Prob. 2.58PCh. 2 - Ethyl butanoate, CH3CH2CH2CO2CH2CH3, is one of the...Ch. 2 - Prob. 2.60PCh. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 2.62PCh. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Prob. 2.64PCh. 2 - Draw the products of each Lewis acid-base...Ch. 2 - Prob. 2.66PCh. 2 - Prob. 2.67PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 2.70PCh. 2 - 2.72 DBU, is a base we will encounter in...Ch. 2 - 2.73 Molecules like acetamide can be protonated...Ch. 2 - Two pKa values are reported for malonic acid, a...Ch. 2 - Prob. 2.74PCh. 2 - 2.76 Write a stepwise reaction sequence using...Ch. 2 - Prob. 2.76PCh. 2 - 2.78 Which compound, M or N, is the stronger acid?...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forwardShow work. don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY