
Concept explainers
Interpretation:
The molecular formula of the given compound has to be determined by using the ideal gas equation and molar mass of that compound. The possible structures of the compound has to be drawn from the determined molecular formula.
Concept Introduction:
Ideal gas equation:
The ideal gas equation is the combined equation of
Explanation of Solution
The given information in the problem is recored as follows:
The given temperature is converted to kelvin as shown here.
The given volume in millilitres is converted into liters by the conversion factor as follows:
Use the below ideal gas equation to find the moles of the vapour.
Substitute as follows.
Use the below equation to find the molar mass of the compound.
Substitute as follows.
The empirical formula of the compound can be calculated as follows:
Assume that the mass of the sample is
The grams of each element is converted into moles by using the molar mass of the corresponding element.
Now the number of moles of each element is converted into whole numbers, dividing by the lowest mole.
Thus, the empirical formula of the compound is
The molecular formula of the compound can be determined from the empirical formula and the molar mass of that compound as follows.
Use the below equation to find the molecular formula.
Substitute as follows.
Hence, the molecular formula of the compound is
The determined molecular formula for the compound is
There are two carbon atoms in the given compound. So, place the two carbon atoms in a straight-line as the parent carbon chain as shown below.
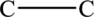
Place the two chlorine atoms on the same carbon atom or for each carbon atom as shown below.
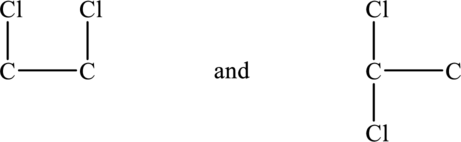
Now add four hydrogen atoms to each carbon atom in order to complete the two structures above because each carbon has four bonds around it. ]
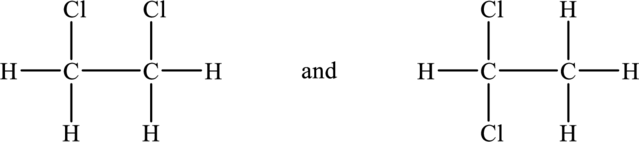
Hence, the possible structures of the compound are show below.
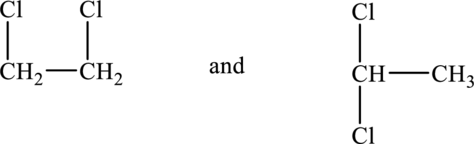
Want to see more full solutions like this?
Chapter 19 Solutions
Foundations of College Chemistry 15e Binder Ready Version + WileyPLUS Registration Card
- Consider a sample of a hydrocarbon at 0.959 atm and 298 K. Upon combusting the entire sample in oxygen, you collect a mixture of gaseous carbon dioxide and water vapor at 1.51 atm and 375 K. This mixture has a density of 1.391 g/L and occupies a volume four times as large as that of the pure hydrocarbon. Determine the molecular formula of the hydrocarbon and name it.arrow_forwardAlcohols A, B and C all have the composition C4H 100. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde; molecules of alcohol B contain a linear carbon chain and can be oxidized to a ketone; and molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone. Write the Lewis structures of these molecules.arrow_forwardTwo of the three isomers of C3H8O are alcohols and one is an ether. Draw Lewis structures for these three isomers.arrow_forward
- The chemical formula C4H10O results in four alcohols and three ethers for a total of seven structuralisomers. Draw pairs of structural formulas for these molecules that illustrate positional and functional isomerism on a sheet of paper. You will be drawing a total of four formulas. Label each pair as positional or functional.arrow_forwardAn unknown substance is found to contain only carbon and hydrogen. It is a liquid that boils at 49 °C at 1 atm pressure. Upon analysis it is found to contain 85.7% carbonand 14.3% hydrogen by mass. At 100 °C and 735 torr, the vapor of this unknown has a density of 2.21 g/L. When it is dissolved in hexane solution and bromine water is added, no reaction occurs. What is the identity of the unknown compound?arrow_forwardWhat are the 9 structural isomers of C4H8Cl2 ? Draw and name each isomersarrow_forward
- Ethanol and dimethyl ether have the same molecular formula C2H6O. Ethanol is liquid at room temperature while dimethyl ether is gas. Kindly elaborate this occurrence.arrow_forwardAn organic compound is analyzed and found to contain 66.7% carbon, 11.2% hydrogen, and 22.1% oxygen by mass. The compound boils at 79.6 °C. At 100 °C and 0.970 atm, the vapor has a density of 2.28 g/L. The compound has a carbonyl group and cannot be oxidized to a carboxylic acid. Suggest a structure for the compound.arrow_forward"Cyclohexene is a linear molecule with 6 carbon Describe the molecule cyclohexene and provide the chemical formula. atoms and 12 hydrogen atoms. There is a triple bond because it ends in - # 4 (Glycerina) ene. The chemical formula is C6H12" Which two functional groups are in the molecule shown below? Use the terms left and right to distinguish them. "The left functional group is a carboxylic acid. The right functional group is an #5 (Trinitress) alcohol." Harrow_forward
- Write the structural formula for propylene glycol, 1,2-propanediol. Why is it classified as an alcohol? Is it a polar molecule? Should it be miscible with water?arrow_forward1. The chemical formula C4H100 results in four alcohols and three ethers for a total of seven structural isomers. Draw pairs of structural formulas for these molecules that illustrate positional and functional isomerism on a sheet of paper. You will be drawing a total of four formulas. Label each pair as positional or functional.arrow_forwarda) What is the molecular formula of a compound that has the empirical formula C3H5O and a molecular mass of 114? b) Give 2 possible structures for the compound – one structure should contain a ring. 2. Draw 10 structural isomers of C5H10O that contain a ring.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





