
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.33QAP
Interpretation Introduction
Interpretation:
The name of the organic compound from the following proton NMR data should be predicted.
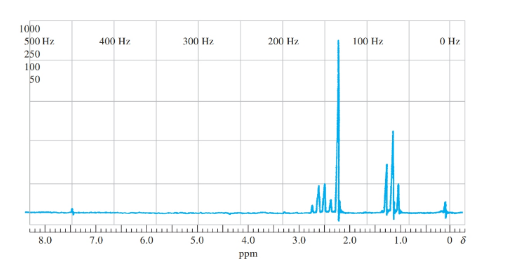
Concept introduction:
Nuclear magnetic resonance is a technique that is used to predict the structural formula of the compound. NMR spectroscopy involves the examination of the nucleus under the external magnetic field.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In a 300 MHz NMR spectrometer, A) what is the
Larmor frequency in MHz of a 15N nucleus? g
H
=
N
26.752; g = 2.7126; B) Using the same NMR
instrument, suppose that a 13C nucleus from a
sample generates a signal which has a frequency
of 11,250 Hz higher than that from the carbons in
TMS. What is the chemical shift of that carbon
atom from the sample?
A) 30 MHz; B) 0.15 ppm
OA) 25 MHz; B) 0.35 ppm
A) 35 MHz; B) 0.30 ppm
OA) 25 MHz; B) 0.55 ppm
What would be the nuclear magnetic resonance spectrum for a proton resonance line that was split by interaction with seven identical protons?
Sketch the form of an A3M2X4 spectrum, where A, M, and X are protons with distinctly different chemical shifts and JAM > JAX > JMX ·
Chapter 19 Solutions
Principles of Instrumental Analysis
Ch. 19 - Prob. 19.1QAPCh. 19 - Prob. 19.2QAPCh. 19 - Prob. 19.3QAPCh. 19 - Prob. 19.4QAPCh. 19 - Prob. 19.5QAPCh. 19 - A nucleus has a spin quantum number of 7/2. How...Ch. 19 - Prob. 19.7QAPCh. 19 - Prob. 19.8QAPCh. 19 - Prob. 19.9QAPCh. 19 - Why is 133C-133C spin-spin splitting not observed...
Ch. 19 - Prob. 19.11QAPCh. 19 - Prob. 19.12QAPCh. 19 - Prob. 19.13QAPCh. 19 - What is a rotating frame of reference?Ch. 19 - How will E for an isolated 13C nucleus compare...Ch. 19 - Prob. 19.16QAPCh. 19 - Prob. 19.17QAPCh. 19 - Prob. 19.18QAPCh. 19 - Prob. 19.19QAPCh. 19 - Prob. 19.20QAPCh. 19 - Prob. 19.21QAPCh. 19 - Prob. 19.22QAPCh. 19 - Prob. 19.23QAPCh. 19 - Prob. 19.24QAPCh. 19 - Prob. 19.25QAPCh. 19 - Prob. 19.26QAPCh. 19 - Prob. 19.27QAPCh. 19 - Prob. 19.28QAPCh. 19 - Prob. 19.29QAPCh. 19 - Prob. 19.30QAPCh. 19 - The proton NMR spectrum in Figure 19.39 is for an...Ch. 19 - The proton NMR spectrum in Figure 19-40 is for a...Ch. 19 - Prob. 19.33QAPCh. 19 - Prob. 19.34QAPCh. 19 - Prob. 19.35QAPCh. 19 - From the proton NMR spectrum in Figure 19-44,...Ch. 19 - From the proton spectrum given in Figure 19-45,...Ch. 19 - Prob. 19.38QAPCh. 19 - Prob. 19.39QAPCh. 19 - Prob. 19.40QAPCh. 19 - Prob. 19.41QAPCh. 19 - Prob. 19.42QAP
Knowledge Booster
Similar questions
- The proton NMR spectrum in Figure 19-40 is for a compound having an empirical formula C4H7BrO2. Identify the compound.arrow_forwardFrom the proton spectrum given in Figure 19-45, determine the structure of this compound, a commc used painkiller; its empirical formula is C10H13NO2.arrow_forwardThe proton NMR spectrum in Figure 19.39 is for an organic compound containing a single atom of bromine. Identify the compound.arrow_forward
- The organic compound 1,4-dimethylbenzene (also known as p-xylene) has the formula (CH3)2C6H4. Its structure has two CH3 (methyl) groups substituted at opposite positions on the benzene (C6H6) ring. Predict the number of peaks in the low-resolution proton NMR spectrum of this compound and the relative areas of the peaks.arrow_forwardShown below is the Proton NMR of a hydrocarbon of the formula C11H16. Given the number of hydrogen atoms represented by each of the 3 integrals, what is the structure of the compound?arrow_forwardPlease look at the Proton NMR spectrum and identify the proposed molecular structure.arrow_forward
- (a) Calculate the energy difference between the two spin states of 1H and of 13C in a magnetic field of 6.8 T. 1H 4.0 1.19e-25 X J 13C 4.0 J (b) What is the precession frequency of a 1H nucleus at this magnetic field? Of a 13C nucleus? 1H 4.0 290 MHz 13C 4.0 73 MHz (c) At what magnetic field do protons precess at a frequency of 300. MHz? 4.0 7.04 Tarrow_forwardIndicate the number of signals of the following molecule in 1H-NMR and the multiplicity of eacu signal (single, doble, multiple, etc.) Then simulate its proton spectre.arrow_forward(a) Calculate the energy difference between the two spin states of 1H and of 13C in a magnetic field of 6.8 T. 1H 4.0 1.91e-29 X J 13C 4.80e-33 X J (b) What is the precession frequency of a 'H nucleus at this magnetic field? Of a 13C nucleus? 1H 4.0 290 MHz 13C 4.0 73 MHz (c) At what magnetic field do protons precess at a frequency of 300. MHz? [4.0 7.04arrow_forward
- We calculated that the resonance frequency of an 1H in the presence of an 11.74 tesla magnet was 500MHz, and we later saw that a 13C nucleus resonantes at approximately 125 MHz in this same magnetic field. What is the resonance frequency of a 19F nucleus in the presence of an 11.74 tesla magnet?arrow_forwardComplete the spectroscopy data tables for a compound with molecular formula C6H14. Determine the structure of the compound. Any labile protons, if they exist, will not be present in this particular 1H NMR spectrum.arrow_forwardDetermine the structure for the molecule that most likely produced these spectraarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning