
Concept explainers
Interpretation: The nuclear symbol of each isotope of sulfur has to be given. The number of neutrons present in each isotope has to be given and the consistency of the
Concept introduction: A different form of the same element is called an isotope.
The
The number of protons present will be equal to the number of electrons.
The number of neutrons is the difference between the mass number and the atomic number.
The average atomic mass of a chemical element is calculated by multiplying the atomic masses of its natural isotopes with their respective abundance.
Answer to Problem 11A
The nuclear symbol of each isotope of sulfur is
The number of neutrons in sulfur-33 is
The number of neutrons in sulfur-34 is
The number of neutrons in sulfur-36 is
No, the average atomic mass of sulfur is in consistence with the relative abundances of the isotopes.
Explanation of Solution
The mass number is the sum of the protons and neutrons in the nucleus.
The atomic number is the number of protons present in the nucleus.
For an element
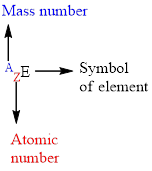
The letter A represents the mass number.
The letter Z represents the atomic number.
Sulfur has an atomic number of 16 but the mass number varies.
For a mass number of 33, the nuclear symbol of the isotope is
For a mass number of 34, the nuclear symbol of the isotope is
For a mass number of 36, the nuclear symbol of the isotope is
The number of neutrons is the difference between the mass number and the atomic number.
For a mass number of 33, the neutrons is
For a mass number of 34, the neutrons is
For a mass number of 36, the neutrons is
The number of neutrons in sulfur-33 is
The number of neutrons in sulfur-34 is
The number of neutrons in sulfur-36 is
The average atomic mass of sulfur is
The atomic mass of sulfur-33 and its relative abundance are
The atomic mass of sulfur-34 and its relative abundance are
The atomic mass of sulfur-36 and its relative abundance are
The average atomic mass can be calculated using the formula,
Average atomic mass=
Average atomic mass=
Average atomic mass=
Average atomic mass=
The average atomic mass of sulfur is not consistent with the relative abundances of the isotopes.
The nuclear symbol of each isotope of sulfur is
The number of neutrons in sulfur-33 is
The number of neutrons in sulfur-34 is
The number of neutrons in sulfur-36 is
No, the average atomic mass of sulfur is in consistence with the relative abundances of the isotopes.
Chapter 19 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





