
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18.2, Problem 18.1P
Interpretation Introduction
(a)
Interpretation:
The IUPAC name of the given compound should be written.
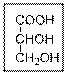
Concept Introduction:
Interpretation Introduction
(b)
Interpretation:
The IUPAC name of the given compound should be written.
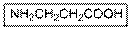
Concept Introduction:
Carboxylic acid group gets priority over
Interpretation Introduction
(c)
Interpretation:
The IUPAC name of the given compound should be written.
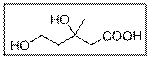
Concept Introduction:
Carboxylic acid group gets priority over alcoholic hydroxyl group.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Problem 18 of 30
Curved arrows are used to illustrate the flow of electrons. Using the provided starting
and product structures, draw the curved electron-pushing arrows for the following
enzyme-catalyzed biological reduction with NADH. Be sure to account for all bond-
breaking and bond-making steps.
:0:
Ï
HH
:0:
NH₂
oi
R
Select to Add Arrows
H
I
Acid
alcohol dehydrogenase
X
H
R
Acid
H
:0:
di
NH₂
N
Submit
Problem 26 of 32
Draw the thiol needed to produce the
sulfide under the conditions shown.
Draw Thiol Reactant
NAH, (CH3)2CHCH2CI
DMSO
Submit
Q
Problem 24 of 26
Draw the product of the reaction shown
below. Ignore inorganic byproducts.
+
Pd(PPH3)4, Na2CO3
benzene
Br
Select to Draw
Submit
Q
Chapter 18 Solutions
Introduction to General, Organic and Biochemistry
Ch. 18.2 - Prob. 18.1PCh. 18.5 - Prob. 18.2PCh. 18.5 - Prob. 18.3PCh. 18 - 18-4 Answer true or false. (a) The functional...Ch. 18 - Prob. 18.5PCh. 18 - 18-6 Name and draw structural formulas for the...Ch. 18 - 18-7 Write the IUPAC name for each carboxylic...Ch. 18 - 18-8 Write the IUPAC name for each carboxylic...Ch. 18 - Prob. 18.9PCh. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Prob. 18.13PCh. 18 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 18 - 18-15 Draw a structural formula for the dimer...Ch. 18 - 18-16 Propanedioic (malonic) acid forms an...Ch. 18 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 18 - 18-18 Propanoic acid and methyl acetate are...Ch. 18 - 18-19 The following compounds have approximately...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - 18-23 Characterize the structural features...Ch. 18 - Prob. 18.24PCh. 18 - Prob. 18.25PCh. 18 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 18 - Prob. 18.27PCh. 18 - 18-28 Arrange these compounds in order of...Ch. 18 - 18-29 Complete the equations for these acid—base...Ch. 18 - 18-30 Complete the equations for these acid-base...Ch. 18 - 18-31 Formic acid is one of the components...Ch. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - 18-41 Complete these examples of Fischer...Ch. 18 - Prob. 18.42PCh. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 18 - 18-47 Methylparaben and propylparaben are used as...Ch. 18 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Prob. 18.51PCh. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - Prob. 18.55P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PROBLEM 18-28(a) Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with ethyleneglycol to give cyclohexanone ethylene acetal.(b) Propose a mechanism for the acid-catalyzed hydrolysis of cyclohexanone ethylene acetal.(c) Compare the mechanisms you drew in parts (a) and (b). How similar are these mechanisms,comparing them in reverse order?arrow_forwardProblem 4.8b Homework Add the appropriate wedges and dashes to make the S enantiomer of 1 - methylcyclohex-2-ene-1 - carboxylic acid. Problem 4.8b Homework Add the appropriate wedges and dashes to make the S enantiomer of 1-methylcyclohex-2-ene-1-carboxylic acid. NN []n + Unanswered. 2 attempts left OH I UZOS - . Brarrow_forwardView Available Hint(s) Hemiacetals Н О 0 HO Acetals CH2OH OH HO OH CH2OH OH OH OH 0 + 0- Neither Reset Helparrow_forward
- 9) Which of the following pairs will form only one product of aldol addition, if the second component will be slowly added to the reaction mixture? Answer A) which CH3 H3C H3C. H3C B) H3C. H3C CH3 H H3C. D) H3C. CH3 H3C H CH3 + H3C CH3 thaich CH3 H3C CH3 l H CH3 Harrow_forwardProblem 27 of 27 Submit Prontosil is an antibacterial sulfa drug. Provide the intermediates, reagents, and product complete this synthesis. Ignore inorganic byproducts. NH2 ΤΟ ON-00 Fe HCI Select to Draw ☐arrow_forwardProblem 3. Write equation showing how 3- could be prepared from Methyl-1,5-pentanediol dicarboxylic acid.arrow_forward
- a a a CO₂H HO H A) HO -H HO -H CH₂OH CH2OH HO -H BA RO B) сон H OH H- -OH CH2OH OH OH -O H C) OCH3 OH OH CO₂H known H- OH D) H- OH H- -OH CO₂H atching (each used once) ace the letter next to the correct description. i) an alditol ii) an aldonic acid iii) an aldaric acid iv) a reducing sugar v) - a glycoside -OH OH -O H OH E) о -0. H OH OH OH OH OHarrow_forward[Review To Use the References to acces What is the IUPAC name of the following? Submit Answer Retry Entire Group 9 more group attempts re.arrow_forwardProblem 27 of 50 Submit Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the missing reactants/ intermediates in this SN1 mechanism. Include all lone pairs and charges as appropriate. Ignore byproducts. Ignore stereochemistry. Select to Draw Alkyl Halide dissociati on CH 02 H H 1,2- hydride shiftarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
