
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
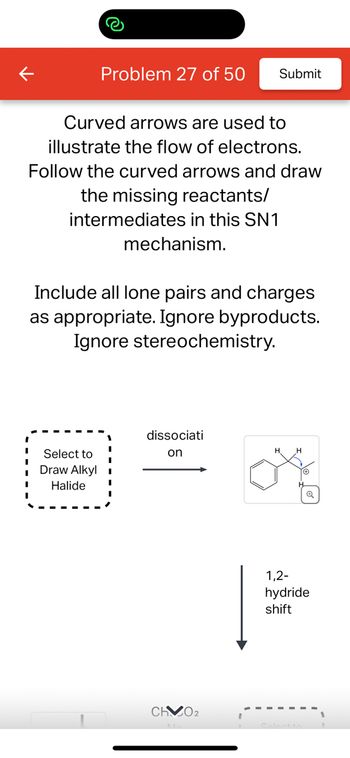
Transcribed Image Text:**Educational Content for SN1 Mechanism**
**Problem 27 of 50**
In this exercise, curved arrows are used to illustrate the flow of electrons within an SN1 reaction mechanism. Your task is to follow these arrows and draw the missing reactants and intermediates.
### Instructions:
1. **Identify the Reactants and Intermediates:**
- Begin by selecting the appropriate alkyl halide. This is the starting point for the SN1 mechanism.
2. **Electron Flow:**
- The curved arrows indicate the direction of electron flow, which you must follow to determine the correct structure of intermediates.
3. **Include Lone Pairs and Charges:**
- Ensure all lone pairs and charges are clearly shown in your drawings.
4. **Ignore Byproducts and Stereochemistry:**
- Focus solely on the main reaction pathway. Byproducts and stereochemical outcomes are not required for this task.
### Diagram Explanation:
- **First Box:** This box prompts you to "Select to Draw Alkyl Halide," indicating the starting molecule before the reaction pathway begins.
- **Dissociation Step:** An arrow labeled "dissociation" leads to the formation of a carbocation intermediate, depicted with a benzene ring and a positively charged carbon center.
- **1,2-Hydride Shift:** This step highlights the migration of a hydrogen atom, with its pair of electrons, from one carbon to an adjacent electron-deficient carbon, resulting in a more stable carbocation.
This exercise is designed to enhance your understanding of electron flow and intermediate species in substitution reactions, specifically within the SN1 mechanism framework.
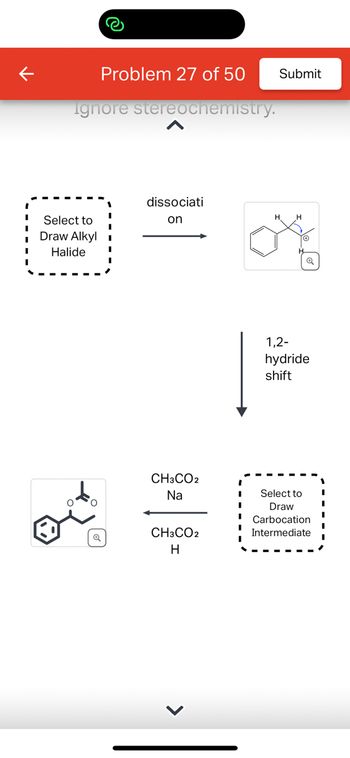
Transcribed Image Text:**Problem 27 of 50**
This diagram illustrates a chemical reaction involving an alkyl halide and subsequent transformations.
1. **Select to Draw Alkyl Halide**: Begin by drawing your chosen alkyl halide structure. This molecule is the starting point for the reaction.
2. **Dissociation**: The alkyl halide undergoes dissociation, resulting in the formation of a carbocation intermediate. The chemical structure shows a six-membered aromatic ring with a positively charged carbon atom, indicating the carbocation.
3. **1,2-Hydride Shift**: This step involves a hydride (H-) shifting from one carbon to the neighboring carbon, stabilizing the carbocation by relocating the positive charge.
4. **Select to Draw Carbocation Intermediate**: Draw the new carbocation structure after the hydride shift. This represents the intermediate stage of the reaction.
5. **Reaction with Reagents (CH₃CO₂Na, CH₃CO₂H)**: The carbocation reacts with sodium acetate (CH₃CO₂Na) and acetic acid (CH₃CO₂H). This transforms the carbocation into the final product.
6. **Formation of Final Product**: The final product is shown with a structure involving an aromatic ring and an ester group, resulting from the combination of reagents and the carbocation intermediate.
This educational example demonstrates key concepts such as carbocation formation, rearrangement, and nucleophilic substitution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help ASAP.arrow_forwardGive detailed mechanism Solution with explanation needed..don't give Handwritten answerarrow_forwardFor 1st picture: which atom is the most nucleophilic? options are A, B or C. For 2nd picture: Identify four electrophilic sites in the molecule? options are give in the pic (choose 4 of them)arrow_forward
- Give detailed mechanism Solution with explanation needed. don't give Ai generated solution. Avoid handwritten Solutionarrow_forwardOwodkdldarrow_forwardved arrows are used to illustrate the flow of electrons. Using the vided starting and product structures, draw the curved electron- shing arrows for the following reaction or mechanistic step(s). sure to account for all bond-breaking and bond-making steps. Drawing Arrows Na NaNHa Select to Add Arrows H H Na H + Undo Reset Donearrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. I :0: Drawing Arrows 0:0 KO H 80 H FA Problem 44 of 50 DII :0: + :O: Undo H Reset ΚΘ Done 0: H Submit *** 100% e E G 1 Draarrow_forwardWhy proposed mechanism is not reasonaarrow_forwardDraw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproducts. Ill!! Av Drawing Problem 26 of 16 Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo X Reset › Drag To Pan Submit Remove Donearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY