
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
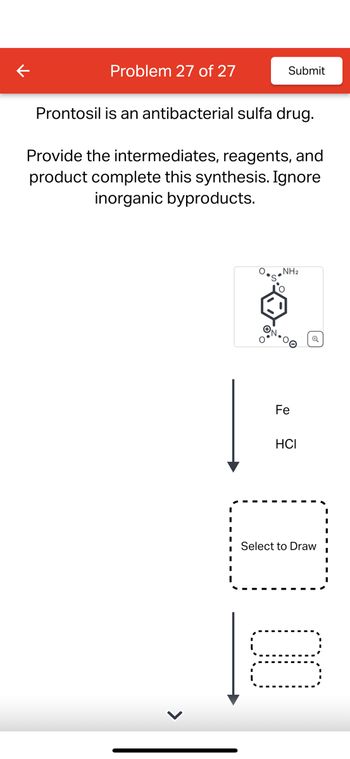
Transcribed Image Text:Problem 27 of 27
Submit
Prontosil is an antibacterial sulfa drug.
Provide the intermediates, reagents, and
product complete this synthesis. Ignore
inorganic byproducts.
NH2
ΤΟ
ON-00
Fe
HCI
Select to Draw
☐
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Amino acids can also be prepared by a two-step sequence that involves Hell–Volhard–Zelinskii reaction of a carboxylic acid followed by treatment with ammonia. Show how you would prepare leucine, (CH3)2CHCH2CH(NH2)CO2H, and identify the mechanism of the second step.arrow_forwardSynthesize 48 assume that the followine also aveilable are acetoacetic ester malonic cstes NHCH3arrow_forwardAs far back as the 16th century, South American Incas chewed the leaves of the coca bush, Erythroxylon coca, to combat fatigue. Chemical studies of Erythroxylon coca by Friedrich Wöhler in 1862 resulted in the discovery of cocaine, C17H21NO4, as the active component. Basic hydrolysis of cocaine leads to methanol, benzoic acid, and another compound called ecgonine, C9H15NO3. Oxidation of ecgonine with CrO3 yields a keto acid that readily loses CO2 on heating, giving tropinone. (a) What is a likely structure for the keto acid? (b) What is a likely structure for ecgonine, neglecting stereochemistry? (c) What is a likely structure for cocaine, neglecting stereochemistry?arrow_forward
- The Ka for dichloroacetic acid is 3.32 Ă— 10-2. Approximately what percentage of the acid is dissociated in a 0.10 M aqueous solution?arrow_forwardAmino acids can be prepared by reaction of alkyl halides with diethyl acetamidomalonate, followed by heating the initial alkylation product with aqueous HCl. Show how you would prepare alanine, CH3CH(NH2)CO2H, one of the twenty amino acids found in proteins, and propose a mechanism for acid-catalyzed conversion of the initial alkylation product to the amino acid.arrow_forwardThe key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete the synthesis?arrow_forward
- The first step in the citric acid cycle of food metabolism is reaction of oxaloacetate with acetyl CoA to give citrate. Propose a mechanism, using acid or base catalysis as needed.arrow_forwardName the following amine, including R, S stereochemistry, and draw the product of its reaction with excess iodomethane followed by heating with Ag2O (Hofmann elimination). Is the stereochemistry of the alkene product Z or E? Explain.arrow_forwardThe diazotization of aniline first involves the formation of NO+ (nitrosonium ion) by the dehydration of nitrous acid with sulfuric acid. The aniline nitrogen then acts as a nucleophile and eventually loses water. Propose a mechanism for the formation of the dizaonium salt of aniline. Use curved arrows to show all electron movement.arrow_forward
- Acetylene is a very weak acid; however, it will react with moist silver(I) oxide and form water and a compound composed of silver and carbon. Addition of a solution of HCl to a 0.2352-g sample of the compound of silver and carbon produced acetylene and 0.2822 g of AgCl. (a) What is the empirical formula of the compound of silver and carbon? (b) The production of acetylene on addition of HCl to the compound of silver and carbon suggests that the carbon is present as the acetylide ion, C22-. Write the formula of the compound showing the acetyl ide ion.arrow_forwardThioglycolic acid, HSCH2CO2H, a substance used in depilatory agents (hair removers) has pKa = 3.42. What is the percent dissociation of thioglycolic acid in a buffer solution at pH = 3.0?arrow_forwardProblem 68 of 80 Submit Draw the product of the reaction shown below. Ignore inorganic byproducts. CI CI Q cat. H2SO4 HNO3 (1 equiv) CI O2N Select to Edit CI >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
