
Concept explainers
Interpretation: The structures of cyclohexa-2, 4-dien-1-one and its enol form are to be written, and the special factors that account for the stability of the enol form are to be identified.
Concept introduction:
Tautomerization is defined as the interconversion of the keto and enol forms. The keto and enol forms are tautomers.
The conjugate base of both the keto and the enol forms is enolate.
If the enolate accepts a proton from carbon, the keto form is formed, and if the enolate accepts a proton from oxygen, the enol form is formed.
Answer to Problem 1PP
Solution:
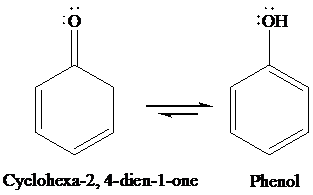
Due to aromaticity.
Explanation of Solution
Under acidic condition, cyclohexa-2, 4-dien-1-one converts to phenol through tautomerism. The structures of cyclohexa-2, 4-dien-1-one and its enol form are as follows:
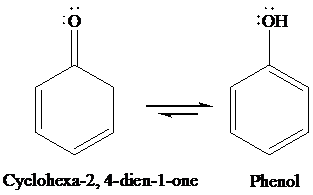
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
