
Concept explainers
Practice Problem 18.6
(a) Write a reaction involving a lithium enolate for introduction of the methyl group in the following compound (an intermediate in a synthesis by E. J. Corey of cafestol, an anti-inflammatory agent found in coffee beans):
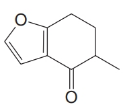
(b) Dienolates can be formed from
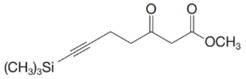
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Living by Chemistry
CHEMISTRY-TEXT
Chemistry: A Molecular Approach
General Chemistry: Atoms First
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Chemistry: The Molecular Nature of Matter
- 17.36 Tamoxifen is an estrogen receptor modulator that is used in the treatment of breast cancer. Provide the missing reagents and the structure of compound A in the synthesis of tamoxifen. Page 707 HO (CH3)2N 1. C,H,MgBr 2. H + Compound A (CH3)2N Tamoxifenarrow_forwardEach of the following reactions has been carried out under conditions such that disubstitution or trisubstitution occurred. Identify the principal organic product in each case. (a) Nitration of p-chlorobenzoic acid (dinitration) (b) Bromination of aniline (tribromination) (c) Bromination of o-aminoacetophenone (dibromination) (d) Bromination of p-nitrophenol (dibromination) (e) Reaction of biphenyl with tert-butyl chloride and iron(III) chloride (dialkylation) (f) Sulfonation of phenol (disulfonation)arrow_forward18.26 Synthesize each of the following compounds from diethyl malonate or ethyl acetoacetate and any other organic and inorganic reagents. (a) (c) (e) OH (b) OH (d) (f)arrow_forward
- Phenylacetone can form two different enols.(a) Show the structures of these enols.(b) Predict which enol will be present in the larger concentration at equilibrium.(c) Propose mechanisms for the formation of the two enols in acid and in basearrow_forwardPractice Problem 18.39 Rank the following compounds in order of increasing reactivity toward electrophilic aromatic substitution. Br A B Br Br E Increasing reactivity toward electrophilic aromatic substitutionarrow_forwardDraw a structural formula for the product formed by treating butanal with each reagent. (a) LiA1H4LiA1H4 followed by H2OH2O (b) NaBH4NaBH4 in CH3OH/H2O (c) H2/Pt (d) Ag(NH3)2+in NH3/H2O (e) H2CrO4, heat (f) HOCH2CH2OH,HClarrow_forward
- Write the structure of the principal organic product obtained on nitration of each of the following: (a) p-Methylbenzoic acid (d) p-Methoxyacetophenone (b) m-Dichlorobenzene (e) p-Methylanisole (c) m-Dinitrobenzene (f ) 2,6-Dibromoanisolearrow_forwardGive the structure of the product formed on reaction of ethyl acetoacetate with each of the following: (a) 1-Bromopentane and sodium ethoxide (b) Saponification (basic hydrolysis) and decarboxylation of the product in part (a) (c) Methyl iodide and the product in part (a) treated with sodium ethoxide (d) Saponification and decarboxylation of the product in part (c) (e) 1-Bromo-3-chloropropane and one equivalent of sodium ethoxide (f) Product in part (e) treated with a second equivalent of sodium ethoxide (g) Saponification and decarboxylation of the product in part (f) (h) Phenyl vinyl ketone and sodium ethoxide (i) Saponification and decarboxylation of the product in part (h)arrow_forwardRank the following compounds in order of increasing acidity, and explain in detail your choice of order.arrow_forward
- Maraviroc, a drug used to treat HIV, is prepared by reductive amination of aldehyde A with amine B. What is the structure of maraviroc, if the most basic N atom of amine B is used in reductive amination?arrow_forwardArrange the following compounds in order of decreasing acidity and explain why?arrow_forwardBoth pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, where as pyrrole is unreactive. What is the best explanation for this observed reactivity.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

