
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.64AP
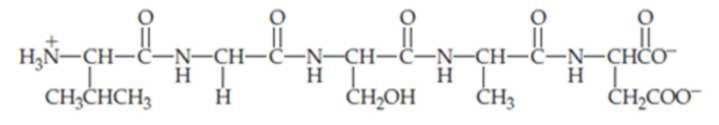
(a) Identify the amino acids present in the peptide shown and name the peptide using the three-letter abbreviations.
(b) Identify the N-terminal and C-terminal amino acids of the peptide.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. don't give Ai generated solution....give correct solution
Biochemistry
What is the process of "transamination" in either the muscles or the liver, that involves keto acid or glutamic acid?
Please explain how the steps work. Thank you!
Biochemistry
Please help. Thank you
What is the importance of glutamic acid in the metabolism of nitrogen from amino acids? (we know therole; it’s used to remove the nitrogen from amino acids so that the remaining carbon skeleton can bebroken down by the “usual” pathways, but what is the important, unique role that only glutamicacid/glutamate can do?)
Chapter 18 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 18.2 - Prob. 18.1PCh. 18.2 - Prob. 18.2PCh. 18.3 - Prob. 18.3PCh. 18.3 - Examine the ball-and-stick model of valine in the...Ch. 18.3 - Indicate whether each of the following molecules...Ch. 18.3 - Prob. 18.6PCh. 18.3 - Prob. 18.7KCPCh. 18.3 - Prob. 18.8PCh. 18.3 - Prob. 18.9PCh. 18.3 - Prob. 18.10P
Ch. 18.3 - Prob. 18.11PCh. 18.3 - Prob. 18.12PCh. 18.4 - The proteins collagen, bovine insulin, and human...Ch. 18.4 - Prob. 18.2CIAPCh. 18.4 - Prob. 18.13PCh. 18.4 - Prob. 18.14PCh. 18.5 - Valine is an amino acid with a nonpolar side...Ch. 18.5 - Tripeptides are composed of three amino acids...Ch. 18.5 - Prob. 18.17PCh. 18.5 - Identify the amino acids in the following...Ch. 18.5 - Prob. 18.19PCh. 18.5 - Prob. 18.3CIAPCh. 18.5 - Prob. 18.4CIAPCh. 18.5 - Two of the most complete (balanced) proteins...Ch. 18.6 - Prob. 18.6CIAPCh. 18.6 - Prob. 18.7CIAPCh. 18.6 - (a)What atoms are present in a planar unit in a...Ch. 18.6 - Prob. 18.21PCh. 18.6 - Prob. 18.22PCh. 18.7 - Prob. 18.23PCh. 18.7 - Prob. 18.24PCh. 18.7 - Complete the following two sentences with either...Ch. 18.7 - Prob. 18.26KCPCh. 18.8 - Which of the following pairs of amino acids can...Ch. 18.8 - Look at Table 18.3 and identify the type of...Ch. 18.8 - In Figure 18.3, identify the amino acids that have...Ch. 18.8 - Prob. 18.30PCh. 18.9 - Prob. 18.31PCh. 18.10 - Another endoprotease is trypsin. Trypsin...Ch. 18.10 - Prob. 18.33PCh. 18.10 - Prob. 18.8CIAPCh. 18.10 - Prob. 18.9CIAPCh. 18 - Draw the structure of the following amino acids,...Ch. 18 - Prob. 18.35UKCCh. 18 - Prob. 18.36UKCCh. 18 - Prob. 18.37UKCCh. 18 - Prob. 18.38UKCCh. 18 - Threonine has two chiral centers. Draw L-threonine...Ch. 18 - Name four biological functions of proteins in the...Ch. 18 - Prob. 18.41APCh. 18 - Prob. 18.42APCh. 18 - Prob. 18.43APCh. 18 - Prob. 18.44APCh. 18 - Prob. 18.45APCh. 18 - Prob. 18.46APCh. 18 - Prob. 18.47APCh. 18 - Draw leucine and identify any chiral carbon atoms...Ch. 18 - Prob. 18.49APCh. 18 - Prob. 18.50APCh. 18 - Is histidine hydrophilic or hydrophobic? Explain...Ch. 18 - Prob. 18.52APCh. 18 - At neutral pH, which of the following amino acids...Ch. 18 - Prob. 18.54APCh. 18 - Prob. 18.55APCh. 18 - Prob. 18.56APCh. 18 - Prob. 18.57APCh. 18 - Proteins are usually least soluble in water at...Ch. 18 - Prob. 18.59APCh. 18 - Prob. 18.60APCh. 18 - Prob. 18.61APCh. 18 - Prob. 18.62APCh. 18 - Prob. 18.63APCh. 18 - (a)Identify the amino acids present in the peptide...Ch. 18 - Prob. 18.65APCh. 18 - Prob. 18.66APCh. 18 - Prob. 18.67APCh. 18 - Prob. 18.68APCh. 18 - Prob. 18.69APCh. 18 - Prob. 18.70APCh. 18 - Prob. 18.71APCh. 18 - Prob. 18.72APCh. 18 - Prob. 18.73APCh. 18 - Prob. 18.74APCh. 18 - Prob. 18.75APCh. 18 - What kind of bond would you expect between chains...Ch. 18 - Is the bond formed between each pair in Problem...Ch. 18 - Prob. 18.78APCh. 18 - Prob. 18.79APCh. 18 - Prob. 18.80APCh. 18 - Prob. 18.81APCh. 18 - Prob. 18.82APCh. 18 - Prob. 18.83APCh. 18 - Prob. 18.84APCh. 18 - Prob. 18.85APCh. 18 - Prob. 18.86APCh. 18 - Prob. 18.87APCh. 18 - Prob. 18.88APCh. 18 - Give an example of a protein that has quaternary...Ch. 18 - Prob. 18.90APCh. 18 - Prob. 18.91APCh. 18 - Prob. 18.92APCh. 18 - Prob. 18.93APCh. 18 - Prob. 18.94APCh. 18 - Prob. 18.95APCh. 18 - Prob. 18.96APCh. 18 - Prob. 18.97APCh. 18 - Prob. 18.98CPCh. 18 - Prob. 18.99CPCh. 18 - Prob. 18.100CPCh. 18 - Prob. 18.101CPCh. 18 - Prob. 18.102CPCh. 18 - Prob. 18.103CPCh. 18 - Prob. 18.104CPCh. 18 - Prob. 18.105CPCh. 18 - Prob. 18.106CPCh. 18 - Prob. 18.107CPCh. 18 - Prob. 18.108CPCh. 18 - Prob. 18.109GPCh. 18 - Prob. 18.110GPCh. 18 - Prob. 18.111GPCh. 18 - Prob. 18.112GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Biochemistry Please help. Thank you When carbamyl phosphate is joined to L-ornathine, where does the energy for the reaction come from?arrow_forwardBiochemistry Question Please help. Thank you What is the function of glutamate dehydrogenase?arrow_forwardBiochemistry Question Please help. Thank you How and why does a high protein diet affect the enzymes of the urea cycle?arrow_forward
- Biochemistry What is the importance of the glucose-alanine cycle?arrow_forwardBiochemistry Assuming 2.5 molecules of ATP per oxidation of NADH/(H+) and 1.5molecules of ATP per oxidation of FADH2, how many ATP are produced per molecule of pyruvate? Please help. Thank youarrow_forward1. How would you explain the term ‘good food’? 2. How would you define Nutrition? 3. Nutrients are generally categorised into two forms. Discuss.arrow_forward
- Biochemistry Question. Please help solve. Thank you! Based upon knowledge of oxidation of bioorganic compounds and howmuch energy is released during their oxidation, rank the following, from most to least, with respect to how much energy would be produced from each during their oxidation. Explain your placement for each one.arrow_forwardBiochemistry Question.For the metabolism of amino acids what is the first step for theirbreakdown? Why is it necessary for this breakdown product to be transported to the liver? For the catabolism of the carbon backbone of these amino acids, there are 7 entry points into the “standard” metabolic pathways. List these 7 entry points and which amino acids are metabolized to these entry points. Please help. Thank you!arrow_forwardBiochemistry Question. Please help. Thank you. You are studying pyruvate utilization in mammals for ATP production under aerobic conditions and have synthesized pyruvate with Carbon #1 labelled with radioactive C14. After only one complete cycle of the TCA cycle, which of the TCA cycle intermediates would be labeled with C14? Explain your answer. Interestingly, you find C14 being excreted in the urine. How does it get there?arrow_forward
- Biochemistry question. Please help with. Thanks in advance For each of the enzymes listed below, explain what the enzyme does including function, names (or structures) of the substrate and products and the pathway(s) (if applicable) it is/are found in. (a) ATP synthetase (b) succinate dehydrogenase (c) isocitrate lyase (d) acetyl CoA carboxylase (e) isocitrate dehydrogenase (f) malate dehydrogenasearrow_forwardDraw and name each alcohol and classify it as primary, secondary, or tertiary. Explain your answer thoroughly.arrow_forwardDraw the product of each reaction. If there are multiple products, draw only the major product. Explain your answer thoroughly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax


Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning


Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax


Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license