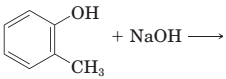Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
11th Edition
ISBN: 9781305705159
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.30P
18-30 Complete the equations for these acid-base reactions.
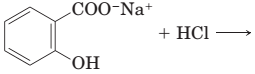
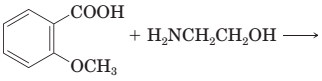
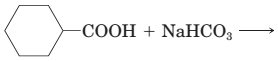
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give detailed mechanism Solution with explanation needed. Don't give Ai generated solution
Show work with explanation needed....don't give Ai generated solution
1.
6. Draw the products for the following reaction:
2.
Diels-Aider
reaction
NOH O
OH
Chapter 18 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 18.2 - Prob. 18.1PCh. 18.5 - Prob. 18.2PCh. 18.5 - Prob. 18.3PCh. 18 - 18-4 Answer true or false. (a) The functional...Ch. 18 - Prob. 18.5PCh. 18 - 18-6 Name and draw structural formulas for the...Ch. 18 - 18-7 Write the IUPAC name for each carboxylic...Ch. 18 - 18-8 Write the IUPAC name for each carboxylic...Ch. 18 - Prob. 18.9PCh. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Prob. 18.13PCh. 18 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 18 - 18-15 Draw a structural formula for the dimer...Ch. 18 - 18-16 Propanedioic (malonic) acid forms an...Ch. 18 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 18 - 18-18 Propanoic acid and methyl acetate are...Ch. 18 - 18-19 The following compounds have approximately...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - 18-23 Characterize the structural features...Ch. 18 - Prob. 18.24PCh. 18 - Prob. 18.25PCh. 18 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 18 - Prob. 18.27PCh. 18 - 18-28 Arrange these compounds in order of...Ch. 18 - 18-29 Complete the equations for these acid—base...Ch. 18 - 18-30 Complete the equations for these acid-base...Ch. 18 - 18-31 Formic acid is one of the components...Ch. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - 18-41 Complete these examples of Fischer...Ch. 18 - Prob. 18.42PCh. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 18 - 18-47 Methylparaben and propylparaben are used as...Ch. 18 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Prob. 18.51PCh. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - Prob. 18.55P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Molecular spectroscopy; Author: Vidya-mitra;https://www.youtube.com/watch?v=G6HjLIWvCQo;License: Standard YouTube License, CC-BY