
Interpretation: The amount of heat required for the conversion of 1 L of water to steam on the Summit of Mount Everest under given conditions should be calculated.
Concept Introduction: The energy required to change the amount of a substance from liquid to gaseous state is said to be the heat of vaporization.
Answer to Problem 98A
The amount of heat required for the conversion of 1 L of water to steam on the Summit of Mount Everest under given conditions is
Explanation of Solution
Given Information:
The molar heat of the vaporization of water at various temperatures is given in the following graph:
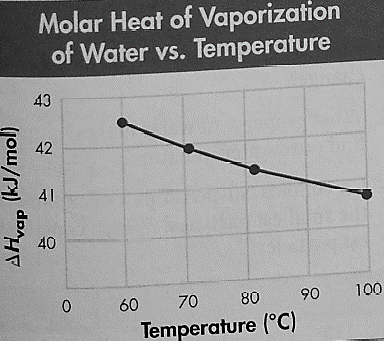
The boiling point on the Summit of Mount Everest is
Converting 1 L to mL:
First, calculate the mass of water using the known value of density of water as 1 g/mL:
Now convert the mass of water to mol, as:
The energy required to vaporize one mole of a liquid at its boiling point is known as the molar heat of vaporization,
It is given that the boiling point on the Summit of Mount Everest is
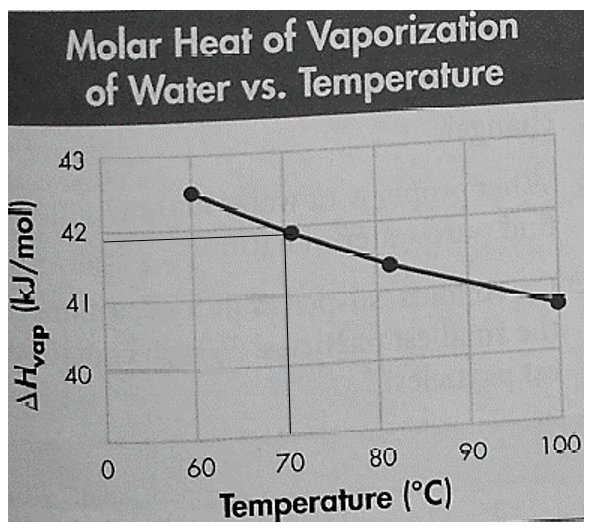
Thus, the value of molar heat vaporization
Hence, the amount of heat required for the conversion of 1 L of water to steam on the Summit of Mount Everest under given conditions is
Chapter 17 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





