
Concept explainers
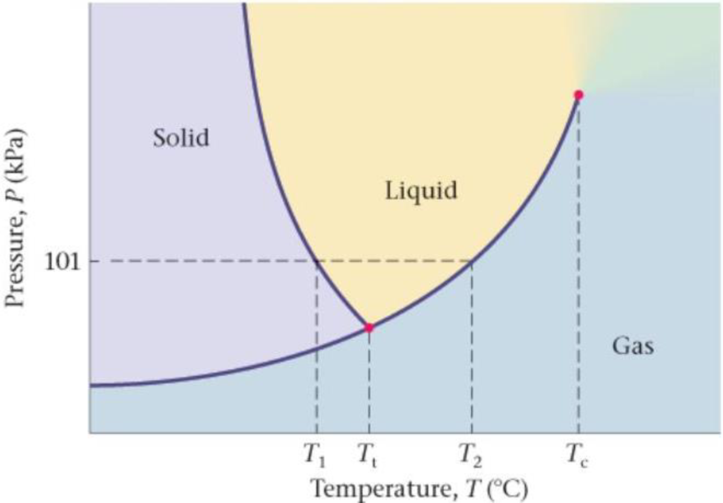
Phase Diagram for Water The phase diagram for water is shown in Figure 17-37. (a) What is the temperature T1 on the phase diagram? (b) What is the temperature T2 on the phase diagram? (c) What happens to the melting/freezing temperature of water if atmospheric pressure is decreased? Justify your answer by referring to the phase diagram (d) What happens to the boiling/condensation temperature of water if atmospheric pressure is increased? Justify your answer by referring to the phase diagram.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Physics (5th Edition)
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Anatomy & Physiology (6th Edition)
Introductory Chemistry (6th Edition)
- Learning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation PV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and I is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, PV = constant. pV T One can see that if the amount of aas remains Figure ЗРО 2po Po 51 Vo 6 2V 3V < 1 of 1 V A W = 2po Vo Submit ✓ Correct ▼ Part D Previous Answers Calculate the work W done by the gas during process 1-3-6. Express your answer in terms of po and Vo. W = 1 1 119225 Templates Symbols undo redo reset keyboard shortcuts ΑΣΦΑ / Submit Request Answer Part E Complete previous part(s) Part F Complete previous part(s) Part G Complete previous part(s) ! L (arrow_forwardLearning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation PV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and I is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, PV = constant. One can see that. if the amount of aas remains Figure ЗРО 2po Po Vo 4 6 2V 3V V 1 of 1 ▼ Calculate the work W done by the gas during process 1-3→6. Express your answer in terms of po and Vo. W = 4po Vo Submit ✓ Correct Part E Calculate the work W done by the gas during process 2→6. Express your answer in terms of po and Vo. VE ΑΣΦ W = Previous Answers Submit Provide Feedback Request Answer Part F Complete previous part(s) Part G Complete previous part(s) ?arrow_forwardLearning Goal: To understand the meaning and the basic applications of PV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation PV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. Figure pV T In this problem, you will be…arrow_forward
- Learning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation PV = nRT where p is the pressure of the gas, V is the volume of the gas, 72 is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change pV To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. 3po In this problem, you will be asked…arrow_forwardSelect all correct statements below to regarding the specific heats_ A. cp and cv are constant values B. dh = cp dT and du = cy dT are true for ideal gases C. cp = cv+R is true for all gases D. cp and cv are functions of temperature only for ideal gasesarrow_forwardA Heating Curve The heating curve shown in the figure is a plot of temperature us. time. It represents the heating of what is Initially ice at --10°C at a constant rate of heat transfer. 110 100. 90 70 60 50 40 30 20. 10 - 10 20 30 50 60 70 80 06 .100 Time (min) Answer ithe following questions, L.a, What phäse or phases are present during segment A? b. What is happering to the energy being absorbed from the heat isource? (Answer in terms of potential and/or kinetie energy.) e. What phane change, f any, is taiding place? 2.a. What phase or phases are present during :Segment B?: b. What la happening to the energy being absorbed? C. 2.a. e. What phase change, if any, is taking place? d.' What is the significance of the temperature 8.a. What phase or phases are present during segment C? b. What le happening to the energy being absorbed? 8. a. e. What phase change, If any, le taking place? 4.a. What phase or phases are present during segment D? 4.a. b. What is happening to the energy belng…arrow_forward
- How much thermal energy needs to be extracted for the temperature to drop to 3x10^2 K? USE IMAGE AS REFERNCEarrow_forwardJ 5arrow_forwardI Review Quantity of heat: Q = cmAT Part A Find what heat in calories (cal) is required to increase the temperature of 60 g water from 0° C to 65 °C. The specific heat capacity of water is 1 cal/g.° C. Express your answer to two significant figures and include the appropriate units. HA Q = Value Units Submit Request Answerarrow_forward
- Please answer the 3 questions. Use the table below the solve the ff thermal expansion problems: An aluminum flagpole is 33 m high. By how much does its length increase as the temperature increases by 15 °C? At 20°C, a brass cube has edge length 30 cm. What is the increase in the surface area when it is heated from 20°C to 75°C? 3. What is the volume of a lead ball at 30.0°C if the ball’s volume at 60.0°C is 50.00 cm3?arrow_forward3 of 4 A stainless-steel-bottomed kettle, its bottom 25 cm in diameter and 1.6 mm thick, sits on a burner. The kettle holds boiling water, and energy flows into the water from the kettle bottom at 800 W. Review I Constants I Periodic Tabl Part A What is the temperature of the bottom surface of the kettle? Thermal conductivity of stainless steel is 14 W/(m K). Express your answer using four significant figures. VO AEO ? T = 102.65 °C Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Review your calculations and make sure you round to 4 significant figures in the last step. Ne Provide Feedbackarrow_forwardPlease try not to reject question? If it is rejected explain why?arrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





