
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 33PCE
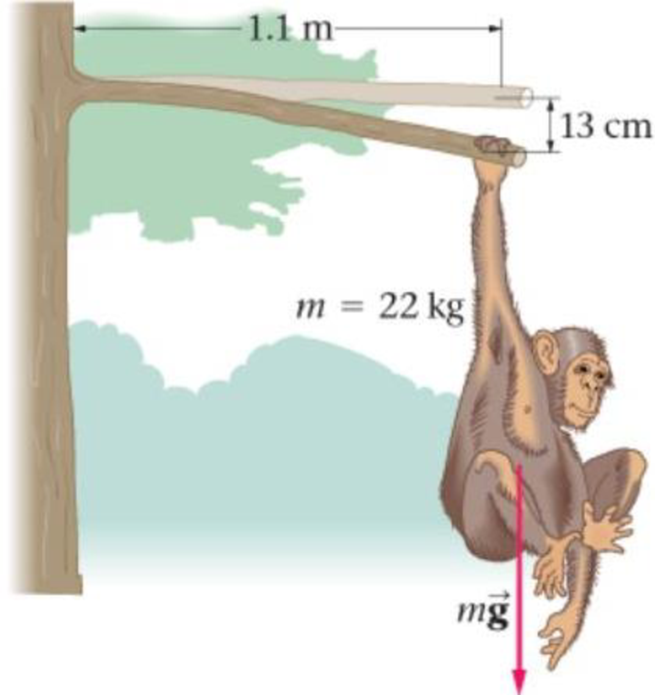
A 22-kg chimpanzee hangs from the end of a horizontal, broken branch 1.1 m long, as shown in Figure 17-34. The branch is a uniform cylinder 4.6 cm in diameter, and the end of the branch supporting the chimp sags downward through a vertical distance of 13 cm. What is the shear modulus for this branch?
Figure 17-34 Problem 33
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
need help on first part
its not 220
No chatgpt pls will upvote
No chatgpt pls
Chapter 17 Solutions
Physics (5th Edition)
Ch. 17.1 - Rank the following ideal-gas systems in order of...Ch. 17.2 - If the Kelvin temperature of a gas is doubled, by...Ch. 17.3 - A metal rod of a given initial length and...Ch. 17.4 - A portion of a substances phase diagram is shown...Ch. 17.5 - Which requires more heat: melting 100 kg of copper...Ch. 17.6 - An ice cube is placed in a cup of water. A few...Ch. 17 - How is the air pressure in a tightly sealed house...Ch. 17 - The average speed of air molecules in your room is...Ch. 17 - Is it possible to change both the pressure and the...Ch. 17 - Prob. 4CQ
Ch. 17 - A camping stove just barely boils water on a...Ch. 17 - An autoclave is a device used to sterilize medical...Ch. 17 - As the temperature of ice is increased, it changes...Ch. 17 - BIO Isopropyl alcohol is sometimes rubbed onto a...Ch. 17 - A drop of water on a kitchen counter evaporates in...Ch. 17 - (a) Is the number of molecules in one mole of N2...Ch. 17 - Predict/Explain If you put a helium-filled balloon...Ch. 17 - Two containers hold ideal gases at the same...Ch. 17 - Prob. 4PCECh. 17 - BIO After emptying her lungs, a person inhales 4.3...Ch. 17 - An automobile tire has a volume of 0.0185 m3. At a...Ch. 17 - Prob. 7PCECh. 17 - A compressed-air tank holds 0.500 m3 of air at a...Ch. 17 - Four ideal gases have the following pressures, P,...Ch. 17 - A balloon contains 3.9 liters of nitrogen gas at a...Ch. 17 - Prob. 11PCECh. 17 - Predict/Calculate A bicycle tire with a volume of...Ch. 17 - A 515-cm3 flask contains 0.460 g of a gas at a...Ch. 17 - Prob. 14PCECh. 17 - The air inside a hot-air balloon has an average...Ch. 17 - Prob. 16PCECh. 17 - Consider the system described in the previous...Ch. 17 - Prob. 18PCECh. 17 - Prob. 19PCECh. 17 - If the translational speed of molecules in an...Ch. 17 - At what temperature is the rms speed of H2 equal...Ch. 17 - Suppose a planet has an atmosphere of pure ammonia...Ch. 17 - Prob. 23PCECh. 17 - Prob. 24PCECh. 17 - Prob. 25PCECh. 17 - What is the temperature of a gas of CO2 molecules...Ch. 17 - The rms speed of a sample of gas is increased by...Ch. 17 - Prob. 28PCECh. 17 - A 380-mL spherical flask contains 0.065 mol of an...Ch. 17 - Prob. 30PCECh. 17 - A rock climber hangs freely from a nylon rope that...Ch. 17 - BIO To stretch a relaxed biceps muscle 2.5 cm...Ch. 17 - A 22-kg chimpanzee hangs from the end of a...Ch. 17 - The Marianas Trench The deepest place in all the...Ch. 17 - Four cylindrical rods with various cross-sectional...Ch. 17 - Predict/Calculate A steel wire 4.1 m long...Ch. 17 - BIO Spiderweb An orb weaver spider with a mass of...Ch. 17 - Predict/Calculate Two rods of equal length (0.55...Ch. 17 - A piano wire 0.82 m long and 0.93 mm in diameter...Ch. 17 - The formation of ice from water is accompanied by...Ch. 17 - Vapor Pressure for Water Figure 17-35 shows a...Ch. 17 - Using the vapor-pressure curve given in Figure...Ch. 17 - Prob. 43PCECh. 17 - Prob. 44PCECh. 17 - Predict/Calculate The Vapor Pressure of CO2 A...Ch. 17 - Phase Diagram for Water The phase diagram for...Ch. 17 - Phase Diagram for CO2 The phase diagram for CO2 is...Ch. 17 - Prob. 48PCECh. 17 - How much heat must be removed from 1.96 kg of...Ch. 17 - A heat transfer of 9.5 105 J is required to...Ch. 17 - How much heat must be added to 2.55 kg of copper...Ch. 17 - An ammonia refrigeration cycle involves the...Ch. 17 - Prob. 53PCECh. 17 - Prob. 54PCECh. 17 - Prob. 55PCECh. 17 - Figure 17-30 shows a temperature-versus-heat plot...Ch. 17 - Predict/Calculate Suppose the 1.000 kg of water in...Ch. 17 - Prob. 58PCECh. 17 - When you go out to your car one cold winter...Ch. 17 - A large punch bowl holds 3.99 kg of lemonade...Ch. 17 - A 155-g aluminum cylinder is removed from a liquid...Ch. 17 - An 825-g iron block is heated to 352 C and placed...Ch. 17 - Party Planning You are expecting to serve 32 cups...Ch. 17 - Predict/Calculate A 35-g ice cube at 0.0 C is...Ch. 17 - A 48-g block of copper at 12 C is added to 110 g...Ch. 17 - A 0 075-kg ice cube at 0.0 C is dropped into a...Ch. 17 - To help keep her barn warm on cold days, a farmer...Ch. 17 - CE As you go up in attitude, do you expect the...Ch. 17 - Prob. 69GPCh. 17 - Prob. 70GPCh. 17 - Prob. 71GPCh. 17 - Cooling Computers Researchers are developing heat...Ch. 17 - Prob. 73GPCh. 17 - Prob. 74GPCh. 17 - Evaporating Atmosphere Hydrogen gas evaporates...Ch. 17 - Prob. 76GPCh. 17 - A Boiling Geyser (a) The column of water that...Ch. 17 - A Melting Glacier (a) A glacier is made of ice of...Ch. 17 - Peter catches a 4 2-kg striped bass on a fishing...Ch. 17 - A steel ball (density=7860kg/m3) with a diameter...Ch. 17 - A lead brick with the dimensions shown in Figure...Ch. 17 - (a) Find the amount of heat that must be extracted...Ch. 17 - Mighty Ice Lift A tremendous force is generated...Ch. 17 - Orthopedic Implants Metals such as titanium and...Ch. 17 - Students on a spring break picnic bring a cooler...Ch. 17 - A 5.9-kg block of ice at 1.5 C slides on a...Ch. 17 - A cylindrical copper rod 37 cm long and 7.5 cm in...Ch. 17 - Prob. 88PPCh. 17 - Prob. 89PPCh. 17 - Prob. 90PPCh. 17 - Prob. 91PPCh. 17 - Referring to Example 17-17 (a) Find the final...Ch. 17 - Referring to Example 17-17 (a) Find the final...
Additional Science Textbook Solutions
Find more solutions based on key concepts
72. Name each molecular compound.
a.
b.
C.
d.
e.
Introductory Chemistry (6th Edition)
WHAT IF? Is allopatric speciation more likely to occur on an island close to a mainland or on a more isolated ...
Campbell Biology (11th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
Researchers cross a corn plant that is pure - breeding forthe dominant traits colored aleurone (C1), full kerne...
Genetic Analysis: An Integrated Approach (3rd Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Children playing in a playground on the flat roof of a city school lose their ball to the parking lot below. One of the teachers kicks the ball back up to the children as shown in the figure below. The playground is 6.10 m above the parking lot, and the school building's vertical wall is h = 7.40 m high, forming a 1.30 m high railing around the playground. The ball is launched at an angle of 8 = 53.0° above the horizontal at a point d = 24.0 m from the base of the building wall. The ball takes 2.20 s to reach a point vertically above the wall. (Due to the nature of this problem, do not use rounded intermediate values-including answers submitted in WebAssign-in your calculations.) (a) Find the speed (in m/s) at which the ball was launched. 18.1 m/s (b) Find the vertical distance (in m) by which the ball clears the wall. 0.73 ✓ m (c) Find the horizontal distance (in m) from the wall to the point on the roof where the ball lands. 2.68 m (d) What If? If the teacher always launches the ball…arrow_forwardIt is not possible to see very small objects, such as viruses, using an ordinary light microscope. An electron microscope can view such objects using an electron beam instead of a light beam. Electron microscopy has proved invaluable for investigations of viruses, cell membranes and subcellular structures, bacterial surfaces, visual receptors, chloroplasts, and the contractile properties of muscles. The "lenses" of an electron microscope consist of electric and magnetic fields that control the electron beam. As an example of the manipulation of an electron beam, consider an electron traveling away from the origin along the x axis in the xy plane with initial velocity ₁ = vi. As it passes through the region x = 0 to x=d, the electron experiences acceleration a = ai +a, where a and a, are constants. For the case v, = 1.67 x 107 m/s, ax = 8.51 x 1014 m/s², and a = 1.50 x 10¹5 m/s², determine the following at x = d = 0.0100 m. (a) the position of the electron y, = 2.60e1014 m (b) the…arrow_forwardNo chatgpt plsarrow_forward
- need help with the first partarrow_forwardA ball is thrown with an initial speed v, at an angle 6, with the horizontal. The horizontal range of the ball is R, and the ball reaches a maximum height R/4. In terms of R and g, find the following. (a) the time interval during which the ball is in motion 2R (b) the ball's speed at the peak of its path v= Rg 2 √ sin 26, V 3 (c) the initial vertical component of its velocity Rg sin ei sin 20 (d) its initial speed Rg √ sin 20 × (e) the angle 6, expressed in terms of arctan of a fraction. 1 (f) Suppose the ball is thrown at the same initial speed found in (d) but at the angle appropriate for reaching the greatest height that it can. Find this height. hmax R2 (g) Suppose the ball is thrown at the same initial speed but at the angle for greatest possible range. Find this maximum horizontal range. Xmax R√3 2arrow_forwardAn outfielder throws a baseball to his catcher in an attempt to throw out a runner at home plate. The ball bounces once before reaching the catcher. Assume the angle at which the bounced ball leaves the ground is the same as the angle at which the outfielder threw it as shown in the figure, but that the ball's speed after the bounce is one-half of what it was before the bounce. 8 (a) Assuming the ball is always thrown with the same initial speed, at what angle & should the fielder throw the ball to make it go the same distance D with one bounce (blue path) as a ball thrown upward at 35.0° with no bounce (green path)? 24 (b) Determine the ratio of the time interval for the one-bounce throw to the flight time for the no-bounce throw. Cone-bounce no-bounce 0.940arrow_forward
- A rocket is launched at an angle of 60.0° above the horizontal with an initial speed of 97 m/s. The rocket moves for 3.00 s along its initial line of motion with an acceleration of 28.0 m/s². At this time, its engines fail and the rocket proceeds to move as a projectile. (a) Find the maximum altitude reached by the rocket. 1445.46 Your response differs from the correct answer by more than 10%. Double check your calculations. m (b) Find its total time of flight. 36.16 x Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate results to at least four-digit accuracy to minimize roundoff error. s (c) Find its horizontal range. 1753.12 × Your response differs from the correct answer by more than 10%. Double check your calculations. marrow_forwardRace car driver is cruising down the street at a constant speed of 28.9 m/s (~65 mph; he has a “lead” foot) when the traffic light in front of him turns red. a) If the driver’s reaction time is 160 ms, how far does he and his car travel down the road from the instant he sees the light change to the instant he begins to slow down? b) If the driver’s combined reaction and movement time is 750 ms, how far do he and his car travel down the road from the instant he sees the light change to the instant he slams on her brakes and car begins to slow down? Please answer parts a-B. Show all work. For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places. DONT FORGET TO DRAW VECTORS! ONLY USE BASIC FORMULAS TAUGHT IN PHYSICS. distance = speed * time.arrow_forwardRace car driver is cruising down the street at a constant speed of 28.9 m/s (~65 mph; he has a “lead” foot) when the traffic light in front of him turns red. a) If the driver’s reaction time is 160 ms, how far does he and his car travel down the road from the instant he sees the light change to the instant he begins to slow down? b) If the driver’s combined reaction and movement time is 750 ms, how far do he and his car travel down the road from the instant he sees the light change to the instant he slams on her brakes and car begins to slow down? c) If the driver’s average rate of acceleration is -9.5 m/s2 as he slows down, how long does it take him to come to a stop (use information about his speed of 28.9 m/s but do NOT use his reaction and movement time in this computation)? Please answer parts a-c. Show all work. For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places unless stated otherwise.…arrow_forward
- How is it that part a is connected to part b? I can't seem to solve either part and don't see the connection between the two.arrow_forwardHello, please help with inputing trial one into the equation, I just need a model for the first one so I can answer the rest. Also, does my data have the correct sigfig? Thanks!arrow_forwardFind the current in the R₁ resistor in the drawing (V₁=16.0V, V2=23.0 V, V₂ = 16.0V, R₁ = 2005, R₂ = and R₂ = 2.705) 2.3052 VIT A www R www R₂ R₂ Vaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
An Introduction to Stress and Strain; Author: The Efficient Engineer;https://www.youtube.com/watch?v=aQf6Q8t1FQE;License: Standard YouTube License, CC-BY