
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.6, Problem 77P
An ammonia–water absorption refrigeration unit operates its absorber at 0°C and its generator at 46°C. The vapor mixture in the generator and absorber is to have an ammonia mole fraction of 96 percent. Assuming ideal behavior, determine the operating pressure in the (a) generator and (b) absorber. Also determine the mole fraction of the ammonia in the (c) strong liquid mixture being pumped from the absorber and the (d) weak liquid solution being drained from the generator. The saturation pressure of ammonia at 0°C is 430.6 kPa, and at 46°C it is 1830.2 kPa.
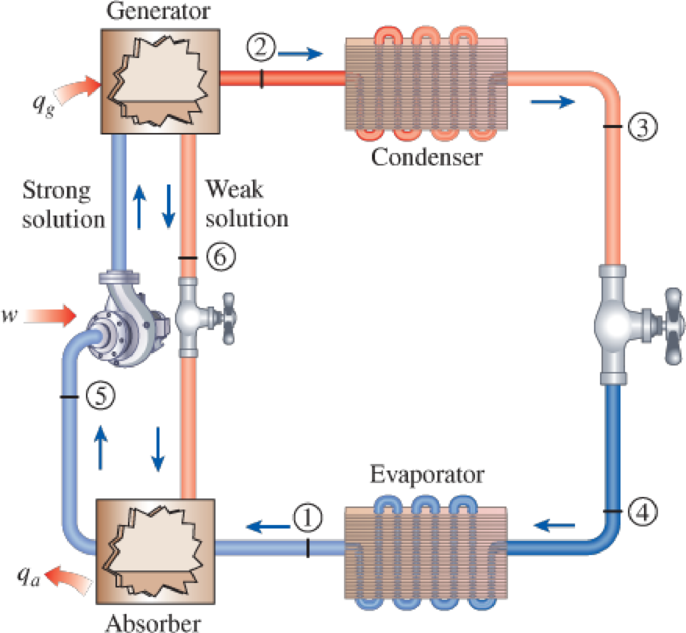
FIGURE P16–77
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
. A bomb calorimeter is used to determine the Higher Caloric Value of an oil sample . the mass of the sample is 2.12g. The total equivalent mass of water is 1.84kg. The increase in temperature after ignition is 8.9K . 2.9g of condensate is formed after it has cooled. Calculate the higher and lower calorific values. The specific heat of water is 4.186kJ/kgk.
3. A product having a moisture content of 60% (wet basis) is dried in a tunnel-type dryer at a rate of 10 kg / hr. The drying air is supplied at a rate of 2000 kg air / hour at 50 ° C and 10% RH, and the dryer exits at 25 ° C, and conditions of equilibrium with the product are at 40% RH. Determine the moisture content of the product leaving the dryer, as well as the water activity of the product. a. Product moisture content =% (wet basis). b. Water activity =
An aqua ammonia absorption plant operates between pressures at 206.8 and 1378 kPaa. The strongsolution enters the generator saturated at 82.2°C and the weak solution leaves the generator saturated at93.3°C. Saturated vapor leaves the generator with a concentration of 0.993 kg NH3 /kg mixture. Assuming thatthe strong solution leaving the absorber is saturated, neglecting pump work and heat losses, calculate perkg/min flow of refrigerant through the evaporator:a. the mass flow of strong solution entering the generator in kg/minb. the mass flow of weak solution leaving the generator in kg/minc. the enthalpy of the weak solution entering the absorber in kJ/kg weak solution and the heat rejected in the absorber in kW
Saturated liquid at 1378 kPaa and 82.2°C has a concentration of 0.46 kg NH3 /kg mixture and an enthalpy of209.2 kJ/kg of mixture, saturated liquid at 1378 kPaa and 93.3°C has a concentration of 0.41 kg NH3 /kg mixtureand an enthalpy of 255.68 kJ/kg of mixture, saturated liquid…
Chapter 16 Solutions
Thermodynamics: An Engineering Approach
Ch. 16.6 - Why is the criterion for chemical equilibrium...Ch. 16.6 - Write three different KPrelations for reacting...Ch. 16.6 - Is a wooden table in chemical equilibrium with the...Ch. 16.6 - A reaction chamber contains a mixture of CO2, CO,...Ch. 16.6 - A reaction chamber contains a mixture of N2and N...Ch. 16.6 - A reaction chamber contains a mixture of CO2, CO,...Ch. 16.6 - Which element is more likely to dissociate into...Ch. 16.6 - Prob. 8PCh. 16.6 - Prob. 9PCh. 16.6 - Prob. 10P
Ch. 16.6 - Prob. 11PCh. 16.6 - Prob. 12PCh. 16.6 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Prob. 15PCh. 16.6 - Prob. 16PCh. 16.6 - Prob. 17PCh. 16.6 - Prob. 18PCh. 16.6 - Prob. 19PCh. 16.6 - Prob. 20PCh. 16.6 - Prob. 21PCh. 16.6 - Prob. 22PCh. 16.6 - Prob. 23PCh. 16.6 - Determine the equilibrium constant KP for the...Ch. 16.6 - Prob. 26PCh. 16.6 - Prob. 27PCh. 16.6 - Carbon monoxide is burned with 100 percent excess...Ch. 16.6 - Prob. 30PCh. 16.6 - Prob. 31PCh. 16.6 - Estimate KP for the following equilibrium reaction...Ch. 16.6 - Prob. 33PCh. 16.6 - A mixture of 3 mol of N2, 1 mol of O2, and 0.1 mol...Ch. 16.6 - Prob. 35PCh. 16.6 - Prob. 36PCh. 16.6 - Prob. 37PCh. 16.6 - Prob. 38PCh. 16.6 - Prob. 40PCh. 16.6 - What is the equilibrium criterion for systems that...Ch. 16.6 - Prob. 43PCh. 16.6 - Prob. 44PCh. 16.6 - Prob. 45PCh. 16.6 - Prob. 47PCh. 16.6 - Prob. 48PCh. 16.6 - Prob. 51PCh. 16.6 - Prob. 52PCh. 16.6 - Prob. 53PCh. 16.6 - Prob. 54PCh. 16.6 - Prob. 55PCh. 16.6 - Prob. 56PCh. 16.6 - Prob. 58PCh. 16.6 - Prob. 59PCh. 16.6 - Prob. 60PCh. 16.6 - Prob. 61PCh. 16.6 - Using the Henrys constant data for a gas dissolved...Ch. 16.6 - Prob. 63PCh. 16.6 - Prob. 64PCh. 16.6 - Prob. 65PCh. 16.6 - Prob. 66PCh. 16.6 - A liquid-vapor mixture of refrigerant-134a is at...Ch. 16.6 - Prob. 68PCh. 16.6 - Prob. 69PCh. 16.6 - An oxygennitrogen mixture consists of 30 kg of...Ch. 16.6 - Prob. 71PCh. 16.6 - Prob. 72PCh. 16.6 - Prob. 73PCh. 16.6 - Prob. 74PCh. 16.6 - Prob. 75PCh. 16.6 - Prob. 76PCh. 16.6 - An ammoniawater absorption refrigeration unit...Ch. 16.6 - Prob. 78PCh. 16.6 - Prob. 79PCh. 16.6 - Prob. 80PCh. 16.6 - One lbmol of refrigerant-134a is mixed with 1...Ch. 16.6 - Prob. 82RPCh. 16.6 - Prob. 83RPCh. 16.6 - Prob. 84RPCh. 16.6 - Prob. 85RPCh. 16.6 - Prob. 88RPCh. 16.6 - Prob. 89RPCh. 16.6 - Prob. 90RPCh. 16.6 - Prob. 91RPCh. 16.6 - Prob. 92RPCh. 16.6 - A constant-volume tank contains a mixture of 1 mol...Ch. 16.6 - Prob. 94RPCh. 16.6 - Prob. 95RPCh. 16.6 - Prob. 96RPCh. 16.6 - Prob. 97RPCh. 16.6 - Prob. 99RPCh. 16.6 - Consider a glass of water in a room at 25C and 100...Ch. 16.6 - Prob. 101RPCh. 16.6 - Prob. 102RPCh. 16.6 - Prob. 105RPCh. 16.6 - Prob. 106RPCh. 16.6 - Prob. 107RPCh. 16.6 - Prob. 108RPCh. 16.6 - Prob. 109FEPCh. 16.6 - Prob. 110FEPCh. 16.6 - Prob. 111FEPCh. 16.6 - Prob. 112FEPCh. 16.6 - Prob. 113FEPCh. 16.6 - Prob. 114FEPCh. 16.6 - Propane C3H8 is burned with air, and the...Ch. 16.6 - Prob. 116FEPCh. 16.6 - Prob. 117FEPCh. 16.6 - The solubility of nitrogen gas in rubber at 25C is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The compression factor of a van deer Waals gas at 100°C and 100 atm given that a= 1.345 atm-L^2/mole^2 and b=0.0322 L/molearrow_forward2. Humid air at 35o C and a pressure of 1 atm and with 98% relative humidity enters a condenser in which the temperature is lowered isobarically (pressure is constant) to 15o C. Part of the water condenses and coexists with the remaining air. The remaining air leaves the condenser flowing through a 20-cm diameter duct at a velocity of 10 m/s. Calculate the rate (moles water/s) at which water condenses.arrow_forwardRP-1 is highly refined form of kerosene used for many first stage rocket engines. The average composition of it is indicated by CH1.9 a. What is the stoichiometric mixture ratio (MR) for RP-1 and oxygen? b. Now, you have a mixture of air and RP-1 with three times more air (in terms of moles) than is needed to burn all the fuel. How high is the final temperature? The heats of formation are given in the table below. In molar quantities, assume 1 mole of air is (O2+3.76N2). The reactants have a temperature of 25°C before combustion. You may use the average values of the specific heats for each constituent. C. Would the adiabatic flame temperature be lower or higher for a rocket engine that uses pure oxygen instead of air? Explain. Constituent Qf kJ/kmol @ 298 K Cp kJ/kmol K CH19 9,358 CO2 (g) -393,522 51.9 O2 (g) 0 34.0 N2 (g) 0 31.6 H₂0 (g) -241.827 40.6arrow_forward
- Problem 13.053 Isobaric Heating A mixture of nitrogen and carbon dioxide has a carbon dioxide mass fraction of 50 percent. This mixture is heated at constant pressure in a closed system from 120 kPa and 30°C to 180°C. Calculate the work produced during this heating in kJ/kg. The universal gas constant is Ru= 8.314 kJ/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties. The work produced during this heating is kJ/kg.arrow_forwardA mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) 1 atm pressure piston cylinder From previous experiments, this chemical reaction is known to absorb 322. kJ of energy. water bath The temperature of the water bath is monitored, and it is determined from this data that 188. kJ of heat flows out of the gases system during the reaction. O exothermic Is the reaction exothermic or endothermic? O endothermic O up Does the temperature of the water bath go up or ? O down down? O neither O in Does the piston move in or out? O out O neither O does work Does the gas mixture do work, or is work done on it? O work done on it O neither How much work is done on (or by) the gas mixture? Be sure your answer has the correct number of…arrow_forwardAir at 90°C and 1.00 atm (absolute) contains 10.0 mole% water. A continuous stream of this air enters a compressor-condenser, in which the temperature is lowered to 15.6°C and the pressure is raised to 3.00 atm. The air leaving the condenser is then heated isobarically to 100°C. Calculate: (a) the fraction of water that is condensed from the air (b) the relative humidity of the air at 100°C (c) and the ratio of the volumetric flow of the outlet air to the volumetric flow of the feed air.arrow_forward
- Problem 13.033 Specific Heat and Molecular Weight The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. Calculate the apparent specific heats and molecular weight of this mixture of gases. The universal gas constant is Ru = 8.314 kJ/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties and the table containing the ideal-gas specific heats of various common gases. The apparent molecular weight of this mixture of gases is The constant-pressure specific heat of the mixture is The constant-volume specific heat of the mixture is kg/kmol. kJ/kg-K. kJ/kg-K.arrow_forwardThe equipment room housing the compressor and condenser of a refrigerant ammonia system has dimensions 5 by 4 by 3 m at 1 bar room pressure. Calculate the minimum mass of the refrigerant in kg which would have to escape into the space to cause a toxic concentration for a 1/2-h exposure.arrow_forwardAir at 90 0C and 1.00 atm (absolute) contains 10.0 mole% water. A continuous stream of this air enters a compressor–condenser, in which the temperature is lowered to 15.6 0C and the pressure is raised to 3.00 atm. The air leaving the condenser is then heated isobarically to 100 0C. Calculate the fraction of water that is condensed from the air, the relative humidity of the air at 100 0C, and the ratio m3 outlet air at 100 0C/m feed air at 90 0C.arrow_forward
- 2 %0 l. Ii. . 7:1Y 5310126247118900636_2 h.w 1- Calculate the dryness fraction (quality) of steam which has 1.5 kg of water in suspension with 50 kg of steam. 2-A vessel having a volume of 0.6 m3 contains 3.0 kg of liquid water and water vapour mixture in equilibrium at a pressure of 0.5 MPa. Calculate (1) Mass and volume of liquid ; (2) Mass and volume of vapour 3-Determine the missing properties and the phase descriptions in the following table for water: T, °C Р, КРа u, kJ/kg Phase description (a) 200 0.6 (Ь) 125 1600 (c) 1000 2950 (d) 75 500 (e) 850 0.0 4-Determine the specific volume of superheated water vapor at 10 MPa and 400°C. 5-A 1.8-m3 rigid tank contains steam at 220°C. Onethird of the volume is in the liquid phase and the rest is in the vapor form. Determine (a) the pressure of the steam, (b) the quality of the saturated mixture. 6- Find the specific volume, enthalpy and internal energy of wet steam at 18 bar, dryness fraction 0.85. 7- Find the dryness fraction, specific…arrow_forward2. 100 mol/hr of an ethanol/H₂O mixture containing 10 mol% ethanol is to be produced as the liquid outlet stream from an isothermal (T-78.15°C) flash unit. The feed stream is 30 mol% ethanol. At 78.15°C the liquid mixture of ethanol(1)/water(2) is adequately described by the following van Laar equations for the activity coefficients: In Y₁ = A12 A21x2 (A₁ A12X1 + A21x2. A12X1 In y2 = A21 A₁2x₁ + A₂1x24 A12X1 2 2 A12 = 1.6798 A21 = 0.9227 Assume that the vapor phase behaves as an ideal gas and that the fugacities of pure ethanol and water are independent of pressure (i.e., that it is appropriate to use modified Raoult's law).arrow_forward1) A stream of outside air is mixed with a stream of return air in an air-conditioning system that operates at standard atmospheric pressure. The flow rate of outdoor air is 1/4 of the mixture flow rate and its condition is 32°C dry bulb temperature and 23°C wet bulb temperature. The flow rate of return air is 1/4 of the mixture flow rate and its condition is 24°C and 50% relativehumidity. Determine the following properties of the mixture: a) enthalpy b) specific humidityc dew paint temperature d) dry bulb temperature e) specific volumearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning
Chemical and Phase Equilibrium; Author: LearnChemE;https://www.youtube.com/watch?v=SWhZkU7e8yw;License: Standard Youtube License