
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16, Problem 51UTC
Interpretation Introduction
Interpretation:
The new nucleus after the emission of a positron needs to be determined.
Concept introduction:
A proton is turned into a neutron and a positron (with a positive charge) leaves the nucleus.
Positron emission happens when a nucleus emits a positron
Expert Solution & Answer
Answer to Problem 51UTC
Solution:
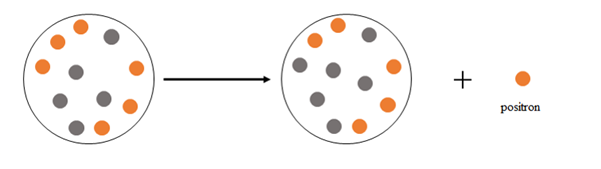
Explanation of Solution
Positron emission happens when a nucleus emits a positron
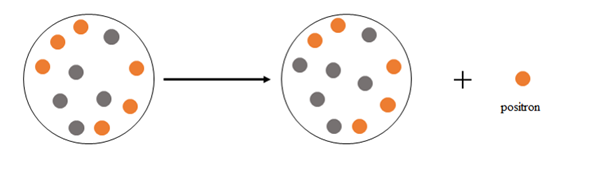
At the beginning there are 6 protons and 5 neutrons, after the emission there are 5 protons and 6 neutrons.
Conclusion
Thus, positron emission happens when a nucleus emits a positron
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
5.62 Write the balanced nuclear equation for
each of the following: (5.1, 5.2)
a. potassium-40 (6 decay
b. sulfur-35 (8 decay)
c. platinum-190 (a decay)
d. Ra-210 (a decay)
e. In-113m (y emission)
5.78 Cesium-137, used in cancer treatment, has a half-life of 30 yr.
(5.2, 5.4)
a. Write the balanced nuclear equation for the beta decay of
cesium-137.
b. How many milligrams of a 16-mg sample of cesium-137
remain after 90 yr?
C. How many years are required for 28 mg of cesium-137 to
decay to 3.5 mg of cesium-137?
The table below is a summary of different modes of nuclear decay. Fill in the changes in atomic number (Z), number of neutrons (N), and mass number (A) in each case. Use “+” sign for increase, “–“ sign for decrease, and “0” for no change. Provide the symbol for each elementary particle involved in the decay process. (12 points)
Decay Mode
Particle Symbol
Change in:
Change in:
Change in:
Z
N
A
Alpha decay
4/2α
Beta decay
0/-1 β
Positron decay
0/+1 β
Electron capture
0/-1 e
Chapter 16 Solutions
Basic Chemistry
Ch. 16.1 - Prob. 1PPCh. 16.1 - Prob. 2PPCh. 16.1 - Naturally occurring potassium consists of three...Ch. 16.1 - Prob. 4PPCh. 16.1 - Prob. 5PPCh. 16.1 - Prob. 6PPCh. 16.1 - Prob. 7PPCh. 16.1 - Prob. 8PPCh. 16.1 - Supply the missing information in the following...Ch. 16.1 - Prob. 10PP
Ch. 16.1 - Prob. 11PPCh. 16.1 - Prob. 12PPCh. 16.2 - Prob. 13PPCh. 16.2 - Prob. 14PPCh. 16.2 - Prob. 15PPCh. 16.2 - Prob. 16PPCh. 16.2 - Prob. 17PPCh. 16.2 - Prob. 18PPCh. 16.2 - Prob. 19PPCh. 16.2 - Prob. 20PPCh. 16.2 - Prob. 21PPCh. 16.2 - Prob. 22PPCh. 16.3 - Prob. 23PPCh. 16.3 - Prob. 24PPCh. 16.3 - Prob. 25PPCh. 16.3 - Prob. 26PPCh. 16.3 - Prob. 27PPCh. 16.3 - Prob. 28PPCh. 16.4 - Prob. 29PPCh. 16.4 - Prob. 30PPCh. 16.4 - Prob. 31PPCh. 16.4 - Prob. 32PPCh. 16.4 - Prob. 33PPCh. 16.4 - Prob. 34PPCh. 16.5 - Prob. 35PPCh. 16.5 - Prob. 36PPCh. 16.5 - Prob. 37PPCh. 16.5 - Prob. 38PPCh. 16.5 - Prob. 39PPCh. 16.5 - Prob. 40PPCh. 16.6 - Prob. 41PPCh. 16.6 - Prob. 42PPCh. 16.6 - Prob. 43PPCh. 16.6 - Prob. 44PPCh. 16.6 - Prob. 45PPCh. 16.6 - Prob. 46PPCh. 16.6 - Prob. 47PPCh. 16.6 - Prob. 48PPCh. 16.6 - Prob. 49PPCh. 16.6 - Prob. 50PPCh. 16 - Prob. 51UTCCh. 16 - Prob. 52UTCCh. 16 - The chapter sections to review are shown in...Ch. 16 - Prob. 54UTCCh. 16 - Prob. 55UTCCh. 16 - Prob. 56UTCCh. 16 - Prob. 57APPCh. 16 - Prob. 58APPCh. 16 - Prob. 59APPCh. 16 - Prob. 60APPCh. 16 - Prob. 61APPCh. 16 - Prob. 62APPCh. 16 - Prob. 63APPCh. 16 - Prob. 64APPCh. 16 - Prob. 65APPCh. 16 - Prob. 66APPCh. 16 - Prob. 67APPCh. 16 - Prob. 68APPCh. 16 - Prob. 69APPCh. 16 - Prob. 70APPCh. 16 - Prob. 71APPCh. 16 - Prob. 72APPCh. 16 - Prob. 73APPCh. 16 - Prob. 74APPCh. 16 - Prob. 75APPCh. 16 - Prob. 76APPCh. 16 - Prob. 77APPCh. 16 - Prob. 78APPCh. 16 - Prob. 79CPCh. 16 - Prob. 80CPCh. 16 - Prob. 81CPCh. 16 - Prob. 82CPCh. 16 - Prob. 83CPCh. 16 - Prob. 84CPCh. 16 - Prob. 85CPCh. 16 - Prob. 86CPCh. 16 - Prob. 87CPCh. 16 - Prob. 88CPCh. 16 - Prob. 89CPCh. 16 - Prob. 90CPCh. 16 - Consider the reaction of sodium oxalate (Na2C2O4)...Ch. 16 - Prob. 34CICh. 16 - Prob. 35CICh. 16 - Prob. 36CICh. 16 - Prob. 37CICh. 16 - Prob. 38CICh. 16 - Prob. 39CICh. 16 - Prob. 40CI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5.64 Complete each of the following nuclear equations: (5.2) a. ? + Co Mn + He 25 27 b. ? N+ e c. e + Kr ? 241 d. He + Am →? + 2,n 2narrow_forwardPart 2: Balancing Nuclear Reactions (5.2) 238 92U. 23Th + He 90 1. Identify the reactants and products in the above reaction. 2. For each element, what do the top (superscripted) and the bottom (subscripted) numbers represent respectively? 3. How many protons and neutrons are found in the uranium isotope used above? 4. How many protons and neutrons are found in the thorium isotope used above? 5. What is the difference in, a. the number of protons between U and Th? b. the number of neutrons between U and Th? c. the mass number between U and Th? 6. How do you explain these differences with respect to the nuclear reaction above?arrow_forwardPart 1: Writing Radioactive Decay Reactions (5.2) lodine-131 (131I) isotope undergoes beta decay. 1. Write the symbol for the beta particle released by iodine-131 (show the respective superscript and subscript values). 2. In the nuclear equation for beta decay of iodine-131, does the beta particle appear on the reactant or product side of the arrow? Why?arrow_forward
- 10.2. Write the equations for the following examples of nuclear decay: a) α emission by Bi 83 193 c) ß+ by 34Se 68 b) ß− by 12Mg 27 d) electron capture by 32Ge 71arrow_forwarda 10.5. Sodium-24 (t½= 15h) is used to study blood circulation. If a patient is injected with aqueous solution of 2ªNaCl whose activity is 2.5 x 10° d/s, how much of the activity present in the patient's body and excreted fluids after 4.0 days? le of 35S a B- emitter, has an 30tiarrow_forwardMacmillan Learning Iridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a patient's body to treat cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while lowering the risk of damage to healthy tissue. Iridium-192 is often used in the head or breast. Use the radioactive decay curve of iridium-192 to answer the three questions. Sample remaining (9) 100 90 70 50- 10 20- 0 time: 10 20 30 10 mass remaining: half-life: 3 70 If the initial sample is 8.00 g. what mass of the original iridium-192 remains after 55 days? Estimate the half-life of the radioisotope. 80 90 100 110 120 130 140 150 160 170 180 190 Time Idays) How many days would it take for two-thirds of the sample to decay? B days daysarrow_forward
- 3.3. Write the nuclear equation for the nuclear reaction in which a nuclei undergoes ß- decay and changes to cesium-133.3.5. Sodium-24 (t1/2= 15h) is used to study blood circulation. If a patient is injected with an aqueous solution of 24NaCl whose activity is 2.5x109d/s, how much of the activity is present in the patient’s body and excreted fluids after 4.0 days?arrow_forwardSW Science 10 Unit 5 Radioactive Decay Worksheet Name:asmin laward th Student #: 5.2.1 Radioactive Decay - Half-Life 1. Complete the following table by shading in the appropriate number of squares or calculating the amount of parent material left or decay product formed. Amount % Decay Product FORMED % Parent Radioactive Mass (g) Fraction of Parent Material Material Lefe Material LEFT original sample 100g 100% 0% after 1 half-life 50g 50% after 2 half-lives 25g 12.5% after 3 half-lives 93.75% after 4 half-lives 2. Use the graph on the following page to plot the results of the table in question #1. NOTE: All Radioactive Decay graphs will have a similar shape.arrow_forward6.7 A neutral K meson decays in flight via KO n*x. If the negative pion is produced at rest, calculate the kinetic energy of the positive pion. [Mass of K° is 498 MeV/c2; that of n is 140 MeV/c?]arrow_forward
- 7.2.5 moles of Ni-56 initially exist. Find the number of moles of Co-56 and Fe-56 after 20 days. (Required nuclide information should be retrieved from https://atom.kaeri.re.kr/nuchart/?zlv=0.)arrow_forwardWatch help video Element X decays radioactively with a half life of 8 minutes. If there are 530 grams of Element X, how long, to the nearest tenth ofa minute, would it take the element to decay to 7 grams? y = a(.5)%arrow_forward(7) Carbon-14 dating is used in archeology to determine the age of items that were alive or were made from living materials. This works because the proportion of radioactive Carbon-14 to the other isotopes of Carbon is relatively constant in the atmo- sphere. (Volcanos constantly refresh the C-14 content.) A wooden tool from an archeological site is found to contain 22 micrograms of C-14, whereas a similar hnount of modern wood would contain 100 micrograms. How old is the tool? 58 surface weat..pdf JoanneCarbonell .pdf PDFarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning