
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15.7, Problem 87P
Liquid octane (C8H18) enters a steady-flow combustion chamber at 25°C and 1 atm at a rate of 0.25 kg/min. It is burned with 50 percent excess air that also enters at 25°C and 1 atm. After combustion, the products are allowed to cool to 25°C. Assuming complete combustion and that all the H2O in the products is in liquid form, determine (a) the heat transfer rate from the combustion chamber, (b) the entropy generation rate, and (c) the exergy destruction rate. Assume that T0 = 298 K and the products leave the combustion chamber at 1 atm pressure.
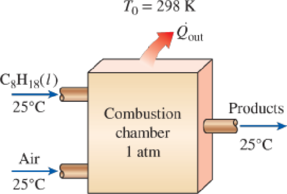
FIGURE P15–87
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part A
The man pulls on the rope with a force of F = 30 N as shown in (Figure 1).
Figure
1.5 m
3 m.
4m
10.5 m
1 of 1
Determine the position vector from O to A.
Express the x, y, and z components of the position vector in meters to three significant figures separated by commas.
ΜΕ ΑΣΦ
vec
(TOA). (TOA)y. (TOA)==
Submit
Request Answer
Part B
m
Determine the position vector from O to B.
Express the x, y, and z components of the position vector in meters to three significant figures separated by commas.
ΜΕ ΑΣΦ
↓↑ vec
(TOB)x, (TOB)y, (TOB) =
Submit
Request Answer
Part C Complete previous part(s)
Provide Feedback
?
m
4
Part A
The tool is used to shut off gas valves that are difficult to access (Figure 1).
Figure
0.25 m
30
0,4 m
<
1 of 1
If the force F= {-60i+40j+15k} N is applied to the handle, determine the component of the moment created about the z axis of the valve.
Express your answer with the appropriate units.
Mz
=
Value
Submit
Request Answer
Provide Feedback
| ?
Units
3. A steam power plant has an average monthly net power delivery of 740 MW over the course of
a year. This power delivery is accomplished by burning coal in the boiler. The coal has a heating
value of 9150 Btu/lbm. The cost of the coal is $14.20/ton. The overall thermal efficiency of the
plant is,
nth
Wnet
Qboiler
0.26 = 26%
Determine the annual cost of the coal required to deliver the given average monthly power.
Chapter 15 Solutions
Thermodynamics: An Engineering Approach
Ch. 15.7 - What are the approximate chemical compositions of...Ch. 15.7 - How does the presence of N2 in air affect the...Ch. 15.7 - Prob. 3PCh. 15.7 - Prob. 4PCh. 15.7 - Is the airfuel ratio expressed on a mole basis...Ch. 15.7 - How does the presence of moisture in air affect...Ch. 15.7 - Prob. 7PCh. 15.7 - Prob. 8PCh. 15.7 - Prob. 9PCh. 15.7 - Are complete combustion and theoretical combustion...
Ch. 15.7 - What does 100 percent theoretical air represent?Ch. 15.7 - Consider a fuel that is burned with (a) 130...Ch. 15.7 - What are the causes of incomplete combustion?Ch. 15.7 - Which is more likely to be found in the products...Ch. 15.7 - Methane (CH4) is burned with the stoichiometric...Ch. 15.7 - Prob. 16PCh. 15.7 - n-Butane fuel (C4H10) is burned with the...Ch. 15.7 - Prob. 18PCh. 15.7 - Propane (C3H8) is burned with 75 percent excess...Ch. 15.7 - Propane fuel (C3H8) is burned with 30 percent...Ch. 15.7 - In a combustion chamber, ethane (C2H6) is burned...Ch. 15.7 - Prob. 22PCh. 15.7 - Prob. 23PCh. 15.7 - Ethane (C2H6) is burned with 20 percent excess air...Ch. 15.7 - Octane (C8H18) is burned with 250 percent...Ch. 15.7 - Prob. 26PCh. 15.7 - A fuel mixture of 60 percent by mass methane (CH4)...Ch. 15.7 - Prob. 28PCh. 15.7 - A certain natural gas has the following volumetric...Ch. 15.7 - Prob. 30PCh. 15.7 - A gaseous fuel with a volumetric analysis of 45...Ch. 15.7 - Prob. 33PCh. 15.7 - The fuel mixer in a natural gas burner mixes...Ch. 15.7 - Prob. 35PCh. 15.7 - Prob. 36PCh. 15.7 - Determine the fuelair ratio when coal from...Ch. 15.7 - Prob. 38PCh. 15.7 - Prob. 39PCh. 15.7 - Prob. 40PCh. 15.7 - Prob. 41PCh. 15.7 - When are the enthalpy of formation and the...Ch. 15.7 - Prob. 43PCh. 15.7 - Prob. 44PCh. 15.7 - Prob. 45PCh. 15.7 - Prob. 46PCh. 15.7 - Prob. 48PCh. 15.7 - Repeat Prob. 1546 for liquid octane (C8H18).Ch. 15.7 - Ethane (C2H6) is burned at atmospheric pressure...Ch. 15.7 - Reconsider Prob. 1550. What minimum pressure of...Ch. 15.7 - Calculate the HHV and LHV of gaseous n-octane fuel...Ch. 15.7 - Prob. 53PCh. 15.7 - Consider a complete combustion process during...Ch. 15.7 - Prob. 56PCh. 15.7 - Prob. 57PCh. 15.7 - Prob. 58PCh. 15.7 - Propane fuel (C3H8) is burned with an airfuel...Ch. 15.7 - Prob. 60PCh. 15.7 - Prob. 61PCh. 15.7 - Prob. 62PCh. 15.7 - Octane gas (C8H18) at 25C is burned steadily with...Ch. 15.7 - Liquid ethyl alcohol [C2H5OH(l)] at 25C is burned...Ch. 15.7 - Prob. 66PCh. 15.7 - A gaseous fuel mixture that is 40 percent propane...Ch. 15.7 - A constant-volume tank contains a mixture of 120 g...Ch. 15.7 - Prob. 70PCh. 15.7 - Prob. 71PCh. 15.7 - Prob. 72PCh. 15.7 - A fuel is completely burned first with the...Ch. 15.7 - Prob. 74PCh. 15.7 - Prob. 75PCh. 15.7 - What is the adiabatic flame temperature of methane...Ch. 15.7 - Octane gas (C8H18) at 25C is burned steadily with...Ch. 15.7 - Acetylene gas (C2H2) at 25C is burned during a...Ch. 15.7 - Ethyl alcohol [C2H5OH(g)] is burned with 200...Ch. 15.7 - Prob. 81PCh. 15.7 - Prob. 82PCh. 15.7 - Reconsider Prob. 1582. The combustion products are...Ch. 15.7 - Express the increase of entropy principle for...Ch. 15.7 - Prob. 85PCh. 15.7 - What does the Gibbs function of formation gf of a...Ch. 15.7 - Liquid octane (C8H18) enters a steady-flow...Ch. 15.7 - Prob. 88PCh. 15.7 - Reconsider Prob. 1588. The automobile engine is to...Ch. 15.7 - Benzene gas (C6H6) at 1 atm and 77F is burned...Ch. 15.7 - Prob. 91PCh. 15.7 - n-Octane [C8H18(l)] is burned in the...Ch. 15.7 - A steady-flow combustion chamber is supplied with...Ch. 15.7 - Prob. 94RPCh. 15.7 - Prob. 95RPCh. 15.7 - Prob. 96RPCh. 15.7 - Prob. 97RPCh. 15.7 - Prob. 98RPCh. 15.7 - Prob. 99RPCh. 15.7 - n-Butane (C4H10) is burned with the stoichiometric...Ch. 15.7 - A gaseous fuel mixture of 60 percent propane...Ch. 15.7 - Calculate the higher and lower heating values of...Ch. 15.7 - Prob. 103RPCh. 15.7 - Methane gas (CH4) at 25C is burned steadily with...Ch. 15.7 - A 6-m3 rigid tank initially contains a mixture of...Ch. 15.7 - Propane gas (C3H8) enters a steady-flow combustion...Ch. 15.7 - Determine the highest possible temperature that...Ch. 15.7 - Liquid propane [C3H8(l)] enters a combustion...Ch. 15.7 - Prob. 109RPCh. 15.7 - Prob. 110RPCh. 15.7 - Prob. 111RPCh. 15.7 - A steam boiler heats liquid water at 200C to...Ch. 15.7 - Repeat Prob. 15112 using a coal from Utah that has...Ch. 15.7 - Liquid octane (C8H18) enters a steady-flow...Ch. 15.7 - Prob. 115RPCh. 15.7 - Consider the combustion of a mixture of an...Ch. 15.7 - Prob. 117RPCh. 15.7 - A fuel is burned steadily in a combustion chamber....Ch. 15.7 - A fuel is burned with 70 percent theoretical air....Ch. 15.7 - Prob. 126FEPCh. 15.7 - One kmol of methane (CH4) is burned with an...Ch. 15.7 - The higher heating value of a hydrocarbon fuel...Ch. 15.7 - Acetylene gas (C2H2) is burned completely during a...Ch. 15.7 - An equimolar mixture of carbon dioxide and water...Ch. 15.7 - A fuel is burned during a steady-flow combustion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The cable exerts a force of P = 4 kN at the end of the 8-m-long crane boom. A P 8 m B -x- I'm En ▾ Part A If 0 = 30°, determine the placement x of the boom at B so that this force creates a maximum moment about point O. Express your answer to three significant figures and include the appropriate units. x = 9.81 m Submit Previous Answers ✓ Correct ▾ Part B What is this moment? Express your answer to three significant figures and include the appropriate units. Assume the positive direction is counterclockwise. (Mo) max 43.7 = E ? N Submit Previous Answers Request Answer X Incorrect; Try Again; 28 attempts remaining Enter your answer with a different unit type. Review a list of acceptable units.arrow_forwardFind highest and lowest temperature.arrow_forwardExplained step by step.arrow_forward
- The bevel gear shown in is subjected to the force F which is caused from contact with another gear. Part A F (201+8j 15k) N 40 mm Determine the moment of this force about the y axis of the gear shaft. Express your answer with the appropriate units. My = Value Submit Request Answer ? Units 30 mmarrow_forwardConsider the beam in. Part A 1.5 ft 200 lb 200lb 2 ft 30° 1.25 ft 30° If F 90 lb, determine the resultant couple moment. = Express your answer in pound-feet to three significant figures. Assume the positive direction is counterclockwise. ΑΣΦ vec MR = Submit Request Answer ? lb.ftarrow_forward4. An operating parameter often used by power plant engineers is the heat rate. The heat rate is defined as, HR Qbioler Wnet where Qbioler is the heat transfer rate (Btu/h) to the water in the boiler due to the combustion of a fuel and Wnet is the net power (kW) delivered by the plant. In comparison, the thermal efficiency of the power plant is defined as, nth Wnet Qbioler where the numerator and denominator have the same units. Consider a power plant that is delivering 1000 MW of power while utilizing a heat transfer rate of 3570 MW at the boiler. Determine the heat rate and thermal efficiency of this power plant.arrow_forward
- The shaft shown in the sketch is subjected to tensile torsional and bending loads Determine the principal stresses at the location of stress concentration ✓ D=45MR F=3MM 1000-M 1000N チ d=30mm 500N 150 мм MM- 120 MA-arrow_forwardcalculate moment of inertia of this tapered beam structurearrow_forwardThe system shown below is in statics equilibrium. Cable OB lies in the xy plane and makes a 30° angle with the positive x-axis. Cable OA lies along the negative y-axis. If the weight of the load being supported is 100 lb, determine the magnitude of the forces in all four cables: OA, OB, OC, and OD.arrow_forward
- This is a mechanics/statics problem involving finding internal reactions, V(x) and M(x). Please refer to image for details. I'm not sure about where to take cuts and how to formulate the equations as a function of x. For my support Reactions I got Ay = 1008.33 lb, By = 1416.67 lb and Cy = 175 lb. and for the first cut V(x) = 1008.33 -250(x) and M(x) = 1008.33x - 125x^2. I'm struggling with the equations for the 2nd and 3rd cut.arrow_forwardAs shown in the figure below, a ring is used to suspend a load and is supported by Cable OA and Spring OB. Given that the tension in Cable OA is 400 N, what is the weight of the load being supported? Assume the system is in static equilibrium.arrow_forward4. (a) State the conditions that must be met to ensure dynamic balance is achieved for long rotors. (b) A rotor carries three out-of-balance discs in planes A, B and C as shown in Figure 4. The out-of- balance mass x radius products of the rotor discs are tabulated in Table 4. The shaft is to be dynamically balanced by adding balancing masses in planes P and Q, spaced along the shaft at a distance da = 800 mm. Determine the magnitude mara and angular position of the balancing mass x radius product that must be added to plane Q. MBB Ов θε mdc Мага End View on Plane P P MBB MATA dA dB dc do Figure 4 moc Table 4 MATA = 0.6 kg mm 6A = 0° d₁ = 200 mm mers = 0.2 kg mm 6g = 45° dB = 400 mm mcrc = 0.4 kg mm Bc=240° dc = 600 mm Ans. (b) = 110.5°, moro = 0.2 kg mmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Extent of Reaction; Author: LearnChemE;https://www.youtube.com/watch?v=__stMf3OLP4;License: Standard Youtube License