
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15.7, Problem 24P
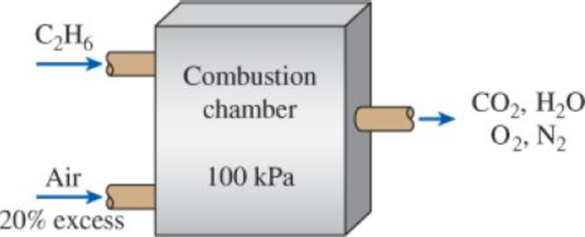
Ethane (C2H6) is burned with 20 percent excess air during a combustion process. Assuming complete combustion and a total pressure of 100 kPa, determine (a) the air–fuel ratio and (b) the dew-point temperature of the products.
FIGURE P15–24
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Chapter 12 - Lecture Notes.pptx: (MAE 272-01) (SP25) DY...
Scores
Consider a large 6-cm-thick stainless steel plate (k = 15.1 W/m-K) in which heat is generated uniformly at a rate of 5 × 105
W/m³. Both sides of the plate are exposed to an environment at 30°C with a heat transfer coefficient of 60 W/m²K.
Determine the value of the highest and lowest temperature.
The highest temperature is
The lowest temperature is
°C.
°C.
Sketch and explain a PV Diagram and a Temperature Entropy Diagram for a 4 stroke diesel engine
please, please explain into detail the difference bewteen the two and referance the a diagram. Please include a sketch or an image of each diagram
Chapter 15 Solutions
Thermodynamics: An Engineering Approach
Ch. 15.7 - What are the approximate chemical compositions of...Ch. 15.7 - How does the presence of N2 in air affect the...Ch. 15.7 - Prob. 3PCh. 15.7 - Prob. 4PCh. 15.7 - Is the airfuel ratio expressed on a mole basis...Ch. 15.7 - How does the presence of moisture in air affect...Ch. 15.7 - Prob. 7PCh. 15.7 - Prob. 8PCh. 15.7 - Prob. 9PCh. 15.7 - Are complete combustion and theoretical combustion...
Ch. 15.7 - What does 100 percent theoretical air represent?Ch. 15.7 - Consider a fuel that is burned with (a) 130...Ch. 15.7 - What are the causes of incomplete combustion?Ch. 15.7 - Which is more likely to be found in the products...Ch. 15.7 - Methane (CH4) is burned with the stoichiometric...Ch. 15.7 - Prob. 16PCh. 15.7 - n-Butane fuel (C4H10) is burned with the...Ch. 15.7 - Prob. 18PCh. 15.7 - Propane (C3H8) is burned with 75 percent excess...Ch. 15.7 - Propane fuel (C3H8) is burned with 30 percent...Ch. 15.7 - In a combustion chamber, ethane (C2H6) is burned...Ch. 15.7 - Prob. 22PCh. 15.7 - Prob. 23PCh. 15.7 - Ethane (C2H6) is burned with 20 percent excess air...Ch. 15.7 - Octane (C8H18) is burned with 250 percent...Ch. 15.7 - Prob. 26PCh. 15.7 - A fuel mixture of 60 percent by mass methane (CH4)...Ch. 15.7 - Prob. 28PCh. 15.7 - A certain natural gas has the following volumetric...Ch. 15.7 - Prob. 30PCh. 15.7 - A gaseous fuel with a volumetric analysis of 45...Ch. 15.7 - Prob. 33PCh. 15.7 - The fuel mixer in a natural gas burner mixes...Ch. 15.7 - Prob. 35PCh. 15.7 - Prob. 36PCh. 15.7 - Determine the fuelair ratio when coal from...Ch. 15.7 - Prob. 38PCh. 15.7 - Prob. 39PCh. 15.7 - Prob. 40PCh. 15.7 - Prob. 41PCh. 15.7 - When are the enthalpy of formation and the...Ch. 15.7 - Prob. 43PCh. 15.7 - Prob. 44PCh. 15.7 - Prob. 45PCh. 15.7 - Prob. 46PCh. 15.7 - Prob. 48PCh. 15.7 - Repeat Prob. 1546 for liquid octane (C8H18).Ch. 15.7 - Ethane (C2H6) is burned at atmospheric pressure...Ch. 15.7 - Reconsider Prob. 1550. What minimum pressure of...Ch. 15.7 - Calculate the HHV and LHV of gaseous n-octane fuel...Ch. 15.7 - Prob. 53PCh. 15.7 - Consider a complete combustion process during...Ch. 15.7 - Prob. 56PCh. 15.7 - Prob. 57PCh. 15.7 - Prob. 58PCh. 15.7 - Propane fuel (C3H8) is burned with an airfuel...Ch. 15.7 - Prob. 60PCh. 15.7 - Prob. 61PCh. 15.7 - Prob. 62PCh. 15.7 - Octane gas (C8H18) at 25C is burned steadily with...Ch. 15.7 - Liquid ethyl alcohol [C2H5OH(l)] at 25C is burned...Ch. 15.7 - Prob. 66PCh. 15.7 - A gaseous fuel mixture that is 40 percent propane...Ch. 15.7 - A constant-volume tank contains a mixture of 120 g...Ch. 15.7 - Prob. 70PCh. 15.7 - Prob. 71PCh. 15.7 - Prob. 72PCh. 15.7 - A fuel is completely burned first with the...Ch. 15.7 - Prob. 74PCh. 15.7 - Prob. 75PCh. 15.7 - What is the adiabatic flame temperature of methane...Ch. 15.7 - Octane gas (C8H18) at 25C is burned steadily with...Ch. 15.7 - Acetylene gas (C2H2) at 25C is burned during a...Ch. 15.7 - Ethyl alcohol [C2H5OH(g)] is burned with 200...Ch. 15.7 - Prob. 81PCh. 15.7 - Prob. 82PCh. 15.7 - Reconsider Prob. 1582. The combustion products are...Ch. 15.7 - Express the increase of entropy principle for...Ch. 15.7 - Prob. 85PCh. 15.7 - What does the Gibbs function of formation gf of a...Ch. 15.7 - Liquid octane (C8H18) enters a steady-flow...Ch. 15.7 - Prob. 88PCh. 15.7 - Reconsider Prob. 1588. The automobile engine is to...Ch. 15.7 - Benzene gas (C6H6) at 1 atm and 77F is burned...Ch. 15.7 - Prob. 91PCh. 15.7 - n-Octane [C8H18(l)] is burned in the...Ch. 15.7 - A steady-flow combustion chamber is supplied with...Ch. 15.7 - Prob. 94RPCh. 15.7 - Prob. 95RPCh. 15.7 - Prob. 96RPCh. 15.7 - Prob. 97RPCh. 15.7 - Prob. 98RPCh. 15.7 - Prob. 99RPCh. 15.7 - n-Butane (C4H10) is burned with the stoichiometric...Ch. 15.7 - A gaseous fuel mixture of 60 percent propane...Ch. 15.7 - Calculate the higher and lower heating values of...Ch. 15.7 - Prob. 103RPCh. 15.7 - Methane gas (CH4) at 25C is burned steadily with...Ch. 15.7 - A 6-m3 rigid tank initially contains a mixture of...Ch. 15.7 - Propane gas (C3H8) enters a steady-flow combustion...Ch. 15.7 - Determine the highest possible temperature that...Ch. 15.7 - Liquid propane [C3H8(l)] enters a combustion...Ch. 15.7 - Prob. 109RPCh. 15.7 - Prob. 110RPCh. 15.7 - Prob. 111RPCh. 15.7 - A steam boiler heats liquid water at 200C to...Ch. 15.7 - Repeat Prob. 15112 using a coal from Utah that has...Ch. 15.7 - Liquid octane (C8H18) enters a steady-flow...Ch. 15.7 - Prob. 115RPCh. 15.7 - Consider the combustion of a mixture of an...Ch. 15.7 - Prob. 117RPCh. 15.7 - A fuel is burned steadily in a combustion chamber....Ch. 15.7 - A fuel is burned with 70 percent theoretical air....Ch. 15.7 - Prob. 126FEPCh. 15.7 - One kmol of methane (CH4) is burned with an...Ch. 15.7 - The higher heating value of a hydrocarbon fuel...Ch. 15.7 - Acetylene gas (C2H2) is burned completely during a...Ch. 15.7 - An equimolar mixture of carbon dioxide and water...Ch. 15.7 - A fuel is burned during a steady-flow combustion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Draw left view of the first orthographic projectionarrow_forwardSketch and Describe a timing diagram for a 2 stroke diesel engine emphasis on the 2 stroke as my last answer explained 4 stroke please include a diagram or sketch.arrow_forwardA 4 ft 200 Ib 1000 Ib.ft C 2 ft 350 Ib - за в 2.5 ft 150 Ib 250 Ib 375 300 Ib Replace the force system acting on the frame. shown in the figure by a resultant force (magnitude and direction), and specify where its line of action intersects member (AB), measured from point (A).arrow_forward
- A continuous flow calorimeter was used to obtain the calorific value of a sample of fuel and the following data collected: Mass of fuel: 2.25 kgInlet water temperature: 11 ° COutlet water temperature 60 ° CQuantity of water: 360 Liters Calorimeter efficiency: 85%Calculate the calorific value of the sample ( kJ / kg ). ive submitted this question twice and have gotten two way different answers. looking for some help thanksarrow_forward15 kg of steel ball bearings at 100 ° C is immersed in 25 kg of water at 20 ° C . Assuming no loss of heat to or from the container, calculate the final temperature of the water after equilibrium has been attained.Specific heat of steel: 0.4857 kJ / kg / ° KSpecific heat of water: 4.187 kJ / kg / ° Karrow_forwardSketch and explain a PV Diagram and a Temperature Entropy Diagram for a 4 stroke diesel enginearrow_forward
- A continuous flow calorimeter was used to obtain the calorific value of a sample of fuel and the following data collected: Mass of fuel: 2.25 kgInlet water temperature: 11 ° COutlet water temperature 60 ° CQuantity of water: 360 Liters Calorimeter efficiency: 85%Calculate the calorific value of the sample ( kJ / kg ).arrow_forwardChapter 12 - Lecture Notes.pptx: (MAE 272-01) (SP25) DY... Scoresarrow_forwardmylabmastering.pearson.com Chapter 12 - Lecture Notes.pptx: (MAE 272-01) (SP25) DY... P Pearson MyLab and Mastering Scoresarrow_forward
- answer the fallowing Brake Specific Fuel Consumption - 0.3 kg/kwh, Mechanical Efficiency- 90% Calorific Value of Fuel -45 MJ/kg. Given these values, find the indicated power, indicated thermal efficiency and brake thermal efficiencyarrow_forwardProblem 6. The circular plate shown rotates about its vertical diameter. At the instant shown, the angular velocity ₁ of the plate is 10 rad/s and is decreasing at the rate of 25 rad/s². The disk lies in the XY plane and Point D of strap CD moves upward. The relative speed u of Point D of strap CD is 1.5 m/s and is decreasing at the rate of 3 m/s². Determine (a) the velocity of D, (b) the acceleration of D. Answers: =0.75 +1.299]-1.732k m/s a=-28.6 +3.03-10.67k m/s² 200 mm x Zarrow_forwardProblem 1. The flywheel A has an angular velocity o 5 rad/s. Link AB is connected via ball and socket joints to the flywheel at A and a slider at B. Find the angular velocity of link AB and the velocity of slider B at this instant. (Partial Answer: @ABN = -2î + 2.25; red Z -1.2 ft C -7 Y -1.5 ft- B 2.0 ftarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Extent of Reaction; Author: LearnChemE;https://www.youtube.com/watch?v=__stMf3OLP4;License: Standard Youtube License