
Concept explainers
When compound C, which is often used to model a more frequently occurring unit in lignins, was ozonized, product D was obtained. In a variety of ways it has been established that the stereochemistry of the three-carbon side chain of such lignin units remains largely if not completely unchanged during oxidations like this.
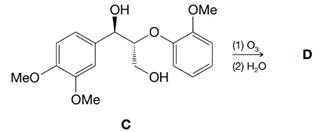
For GC/MS, D was converted to its tetrakis(O-trimethylsilyl) derivative, which had
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Fundamentals of Heat and Mass Transfer
Living By Chemistry: First Edition Textbook
General Chemistry: Atoms First
Chemistry: The Central Science (13th Edition)
Introductory Chemistry (6th Edition)
Chemistry For Changing Times (14th Edition)
- Treatment of compound C (molecular formula C9H12O) with PCC affords D (molecular formula C9H10O). Use the 1H NMR and IR spectra of D to determine the structures of both C and D.arrow_forward-Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardThe base-promoted rearrangement of an -haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. It is proposed that NaOH first converts the a-haloketone to the substituted cyclopropanone shown in brackets and then to the sodium salt of cyclopentanecarboxylic acid. (a) Propose a mechanism for base-promoted conversion of 2-chlorocyclohexanone to the proposed intermediate. (b) Propose a mechanism for base-promoted conversion of the proposed intermediate to sodium cyclopentanecarboxylate.arrow_forward
- Two products, A and B, are obtained from the reaction of 1-bromobutane with NH3. Compound A reacts with acetyl chloride to form C, and compound B reacts with acetyl chloride to form D. The IR spectra of C and D are shown. Identify A, B, C, and D.arrow_forwardDiisopinocampheylborane (Ipc2BH) is a chiral organoborane, readily employed for the production of many asymmetric products used in total synthesis. It is a crystalline material that can be prepared as a single enantiomer via the hydroboration of two equivalents of α-pinene with borane.Explain why only one enantiomer of Ipc2BH is formed.arrow_forwardReaction of (CH3)3CCHO with (C6H5)3P=C(CH3)OCH3, followed by treatment with aqueous acid, affords R (C7H14O). R has a strong absorption in its IR spectrum at 1717 cm−1 and three singlets in its 1H NMR spectrum at 1.02 (9 H), 2.13 (3 H), and 2.33 (2 H) ppm. What is the structure of R? We will learn about this reaction in Chapter 18.arrow_forward
- (10pts) Compound A, C10H16, was found to be optically active. On catalytic reduction over a palladium catalyst, 2 equivalents of hydrogen were absorbed, yielding compound B, CioH2o. On ozonolysis of A, two fragments were obtained. One fragment was identified as acetic acid (CHCOOH). The other fragment, compound C, was an optically active carboxylic acid, C8H14O2. Write reactions, and draw the correct structures for A-C, explain your answer in detail.arrow_forwardDraw the structural formulas for the first two intermediates formed in the hydrolysis of (1R,2S)-1,2-epoxy-1-methylcyclopentane with aqueous acid. Show stereochemistry if product is a meso compound.arrow_forwardThe reaction of 1- methylcyclopentene with mercuric trifluoroacetate in ethanol followed by reduction with sodium borohydride gives 1-ethoxy-1- methylcyclopentane. The -OH peak in the IR shows up at 1700 cm-1 as a sharp peak. (S)-2-bromohexane is the product of the reaction of (S)-2-hexanol with PBr3. The 1H NMR spectrum of 1- pentanol will show a peak for the H atom of the -OH group at 10 ppm delta. A tosylate is a great protecting group for an alcohol. The Williamson ether synthesis is an SN2 reaction of an alkoxide ion with a primary alkyl halide. Thionyl chloride converts ketones into alkyl halides. In an oxidation reaction, the oxygen content of our organic molecule decreases. ✓ [Choose ] False True [Choose ] [Choose ] [Choose ] [Choose ] [Choose ] [Choose ] [Choose ]arrow_forward
- The p-toluenesulfonate derived from (R)-2-octanol and p-toluenesulfonyl chloride was allowed to react with sodium benzenethiolate (C6H5SNa). Give the structure, including stereochemistry and the appropriate R or S descriptor, of the product.arrow_forward4.35 Formulate the reaction of cyclohexene with (i) Br2 and (ii) meta-chloro- peroxybenzoic acid followed by H30+. Show the reaction intermediates and the final products with correct cis or trans stereochemistry. 4.36 What products would you expect to obtain from reaction of cyclohexa- 1,3-diene with each of the following? (a) 1 mol Br2 in CH2C12 (c) 1 mol DCl (D = deuterium, ²H) (b) 1 mol HCl (d) 2 mol H2 over a Pd catalyst 4.37 Predict the products of the following reactions on hex-1-yne: (a) 1 equiv HBr ? (b) 1 equiv Cl2 ? (c) H2, Lindlar catalystarrow_forwardCompound A has the molecular formula C14H25Br and was obtained by reaction of sodium acetylide (HC≡CNa) )with 1,12-dibromododecane. On treatment of compound A with sodium amide, it was converted to compound B (C14H24). Ozonolysis of compound B gave the diacid HO2C(CH2)12CO2H. Catalytic hydrogenation of compound B over Lindlar palladium gave compound C (C14H26), while hydrogenation over platinum gave compound D (C14H28). Sodium-ammonia reduction of compound B gave compound E (C14H26). Both C and E yielded O═CH(CH2)12CH═O on ozonolysis. Assign structures to compound A through E so as to be consistent with the observed transformations.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

