
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 53UTC
The chapter sections to review are shown in parentheses at the end of each problem.
15.53 Consider the following voltaic cell: (15.3)
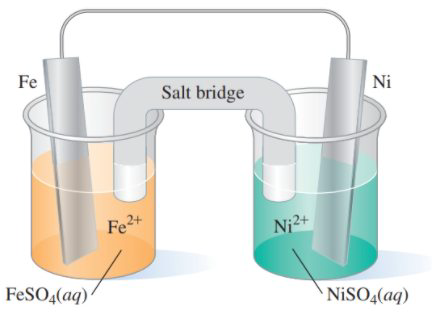
a. What is the oxidation half-reaction?
b. What is the reduction half-reaction?
c. What metal is the anode?
d. What metal is the cathode?
e. What is the direction of electron flow?
f. What is the overall reaction that takes place?
g. Write the shorthand cell notation.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(7.8) A voltaic cell is based on Co*2/Co half-
cell and a AgCl/Ag half-cell.
a) What reaction occurs at the anode?
b) What is the standard cell potential,
E°cell ?
(a) Choices: The reaction that occurs at the
anode:
(A) Co*2(aq) + 2e¯ ® Co(s)
(B) Co(s) ® Co²(aq)
+2,
+ 2e-
(C) Ag*(aq) + e¯
® Ag(s)
(D) Ag(s) ® Ag* (aq) + e¯
Answer: The reaction that occurs at the
anode is
(CAPITAL
LETTER only).
b) What is the standard cell potential,
E°cell ?
Choices: (A) +0.52 V
(C) -0.52 V
(B) -1.08 V
(D) +1.08 V
Answer: The standard cell potential of the
cell, E°cell =
V.
Refer to the Table of Standard Reduction
Potential below.
STANDARD REDUCTION POTENTIAL AT 25 °C
Retie
Hall eaction
Ag'ay+e Agls)
Agle) Ag) + r"(ag)
AgCs) +- Ag)
0.0 2 H0)+ 2e Hl)+ 20H Tng
0.10 HO, (a) + HAXn + 2e 3OH(a)
+0.22 HO) + 2H() + 2e-2H0
-0. Hea 2 2 Ha
0.45ag (a) + te He"(
AgCN, (a+ r- Ag) + 2CN (a)
Ag:CrO) 2e 2Ag) Co/
Aglis - Ag +F)
+0.79
AgSOl - Ag) + 25,0 (ag)
A la+ 3e - AA
als) + 2e 2(a)
+1.20
Ha ag + 2() + 2- HO + H,0)…
(7.7)
A voltaic cell based on the half-
reactions:
In (aq)
– In*3(ag) + 2e™
(aq)
Br2() +2e¯→ 2Br¯(ag)
The standard emf for this cell is +1.46 V.
Using the data below, calculate, E'red for
the reduction of In*3 to In*1.
Br2() + 2e¯ → 2B1¯(ag) (cathode)
E red(cathode) = E
%3D
Br(aq) = +1.06 V
(See Table below)
Choices:
(A) +0.40 V
(B) -0.40 V
(C) + 2.50 V
(D) -2.50 V
Answer: E'red for the reduction of
In+3 =
to In*1
(in
volts)
STANDARD REDUCTION POTENTIALS at 25°C;
Reduction Half Reaction
F: (g) + 2e → 2F (aq)
HO, (aq) + 2H" (aq) + 2e 2H;0
PbO(3) +4H" (ag) + So (ag) + 2e PbSO(s) + HO
MnO. (aq) + 8H" (aq)+ Se+ Ma" (ag) + 4H,o
Au" (aq) + 3e Au (s)
Ch (g) + 2e 2C (ag)
CrO (aq) + 14H" (aq) + 6e →2Cr" (aq) + TH:O
MnO: (3) i 4H" (aq) 2e Ma (aq) 2H:0
O: (g) + 4H" (aq) + 4e + 2H:0
Br: () +2e + 2Br (ag)
NO, (aq) + 4I" (aq) + 3e + NO (g) + 2H:0
2Hg (aq) + 2e Hg" (aq)
Hgr" (aq) + 2e→ 2Hg ()
Ag (aq) +e Ag (s)
Fe (aq) + e Fe (aq)
O: (g) - 21 (aq) + 2e HO: (ag)
E MnO" (aq) + 2H0 + 3e MnO: (3) + 40H…
(7.3) Write the half-cell reactions for each of
the following cells. Choose from the given
options the BEST answer. Write the
CAPITAL LETTER of your cholce.
(a) Ag I Ag* a H* I Hz 1 Pt
Answer:
Half-cell reactions: (A) ) & ()
(B) ) & (iv) (C) (i) & () (D)
(i) & (iv)
O Agʻ(aq) o Agts) + e
Ci) Agts) Ag'laq) +
Clii) 2 H"(aq, IM)
2e" - Hzg 1 atm)
(iv) Hag 1 atm) - 2 H'taq,
IM) * 2e
I Brzn PE
Answer:
Hall-cell reactions: (w W& Cii) (B)
(im & (i) (C) (1) & (iv)
& (iv)
(D) (i)
Crz0, ng • 14 H'cag) *• 6e
Ge 2 Cr*tag) + 7 H,0D
(ii) 2 Br 2e O Brzn
(iv) Brzo 2 Br+ 2e
Chapter 15 Solutions
Basic Chemistry
Ch. 15.1 - Identify each of the following as an oxidation or...Ch. 15.1 - Identify each of the following as an oxidation or...Ch. 15.1 - Prob. 3PPCh. 15.1 - Prob. 4PPCh. 15.1 - Prob. 5PPCh. 15.1 - Prob. 6PPCh. 15.1 - Prob. 7PPCh. 15.1 - Prob. 8PPCh. 15.1 - Prob. 9PPCh. 15.1 - Prob. 10PP
Ch. 15.1 - Prob. 11PPCh. 15.1 - Prob. 12PPCh. 15.1 - What is the oxidation number of the specified...Ch. 15.1 - What is the oxidation number of the specified...Ch. 15.1 - Prob. 15PPCh. 15.1 - Prob. 16PPCh. 15.1 - Prob. 17PPCh. 15.1 - Prob. 18PPCh. 15.1 - Prob. 19PPCh. 15.1 - Prob. 20PPCh. 15.2 - Balance each of the following half-reactions in...Ch. 15.2 - Prob. 22PPCh. 15.2 - Prob. 23PPCh. 15.2 - Use the half-reaction method to balance each of...Ch. 15.2 - Use the half-reaction method to balance each of...Ch. 15.2 - Use the half-reaction method to balance each of...Ch. 15.3 - Use the activity series in Table 15.3 to predict...Ch. 15.3 - Use the activity series in Table 15.3 to predict...Ch. 15.3 - Prob. 29PPCh. 15.3 - Prob. 30PPCh. 15.3 - Prob. 31PPCh. 15.3 - Prob. 32PPCh. 15.3 - The following half-reaction takes place in a...Ch. 15.3 - The following half-reaction takes place in a...Ch. 15.3 - The following half-reaction takes place in a...Ch. 15.3 - Prob. 36PPCh. 15.4 - What we call "tin cans" are really iron cans...Ch. 15.4 - Prob. 38PPCh. 15.4 - Prob. 39PPCh. 15.4 - Prob. 40PPCh. 15.4 - Prob. 41PPCh. 15.4 - Prob. 42PPCh. 15 - Prob. 43UTCCh. 15 - Prob. 44UTCCh. 15 - Prob. 45UTCCh. 15 - Prob. 46UTCCh. 15 - The chapter sections to review are shown in...Ch. 15 - The chapter sections to review are shown in...Ch. 15 - The chapter sections to review are shown in...Ch. 15 - Prob. 50UTCCh. 15 - Prob. 51UTCCh. 15 - Prob. 52UTCCh. 15 - The chapter sections to review are shown in...Ch. 15 - The chapter sections to review are shown in...Ch. 15 - Which of the following are oxidation-reduction...Ch. 15 - Which of the following are oxidation-reduction...Ch. 15 - In the mitochondria of human cells, energy is...Ch. 15 - Prob. 58APPCh. 15 - Prob. 59APPCh. 15 - Prob. 60APPCh. 15 - Prob. 61APPCh. 15 - Prob. 62APPCh. 15 - Prob. 63APPCh. 15 - Write the balanced half-reactions and a balanced...Ch. 15 - Prob. 65APPCh. 15 - Use the activity series in Table 15.3 to predict...Ch. 15 - Prob. 67APPCh. 15 - Prob. 68APPCh. 15 - Prob. 69APPCh. 15 - Prob. 70APPCh. 15 - Prob. 71APPCh. 15 - In an acidic dry-cell battery, the following...Ch. 15 - Steel bolts made for sailboats are coated with...Ch. 15 - Copper cooking pans are stainless steel pans...Ch. 15 - Prob. 75CPCh. 15 - Prob. 76CPCh. 15 - Prob. 77CPCh. 15 - The following problems are related to the topics...Ch. 15 - The following problems are related to the topics...Ch. 15 - The following problems are related to the topics...Ch. 15 - Prob. 81CPCh. 15 - Prob. 82CPCh. 15 - Prob. 83CPCh. 15 - Prob. 84CPCh. 15 - Prob. 85CPCh. 15 - Prob. 86CPCh. 15 - Prob. 87CPCh. 15 - Prob. 88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the blanks: (7.14) In the electrolysis of CUSO4, how much copper is plated out on the cathode by a current of a 0.75A in 10 minutes? (Cu 63.546 g/mol) Analysis/Strategy: ampere & time coulombs ® mol e¯ (or Faraday) ® mol Cu (R mass of Cu Reduction half-reaction of copper solution to copper metal: Cu*²(aq) + e¯ ® Cu(s) copper solution, CUSO4, has the ions: [Cu*2 SOq] Answer: The mass of copper plated out is g Cu. (3 sig fig)arrow_forward(7.9) Using potentials listed in the Table, the standard reduction determine whether the following reaction is spontaneous under standard conditions. Support your answer. Hg*2(aq) + 21¯(aq) → ,+2, Hga) +I2(s) From the Table: Hg*2(aq) + 2e¯ → Hga) E°red = +0.85 V I2(s) + 2e- - 21 (aq) E°red = +0.54 V Answer: Is the above reaction spontaneous? (YES or NO) Briefly support your answer (. Refer to the Table of Standard Reduction Potential below. STANDARD REDUCTION POTENTIAL AT 25 °C ETV) atteaction +0.50 2H,O + 2e-Hlg) + 20H (a Hait-Reaction (V) Ag (ag) +- Agis) Aglle(s)+- Ag)+ r (a) AgCIs) - Ag(s) -a(ap) Ag(CN), (a) eAgs)+ 2CN (a) -O83 Agi+ r(a) 0.10 HO, (a) + HO) + 2e 3OH (a) +0.22 HOa) + 2H(ag) 2e 2Ho) -0.31 He(a 2e 2 Hg) +0.45 2Hg"(a)+ 2e Hg () -0.15 He(a)+ 2e Hel) ) + 2e 21(a) -1.46 2 10, (a) + 12H(a) + 10e l) +6HO) +0.56K'(ag) K) +1.78 a79 AgCro,(s) + 2e 2 Ags) + Cro ) Agl(s) +e- Ag) (a) AgrS,0(a) + - Agis) + 25,0 (a) A" (a) + 3e - A) 92 054 1.20 HAs0,n- 2H"(a) + 2e HAOa) + H,o)…arrow_forwardFill in the blanks: (7.13) Using a current of 4.75 A, how many minutes does it take to plate a sculpture with 1.50 g of Cu from a CuS04 solution? (Cu 63.546 g/mol) Analysis/Strategy: mass of Cu ® mol Cu ® mol e" (or Faraday) ® coulombs ® time in minutes (given current in amperes) Reduction half-reaction of copper solution to copper metal: Cu*2(aq) · + e¯ ® Cu(s) copper solution, CUSO4, has the ions: [Cu*2_SOq] Answer: The time needed to plate out a sculpture is minutes. (3 sig fig)arrow_forward
- Fill in the blanks: (7.6) Describe in shorthand notation a galvanic cell for which the cell has the following reaction: Cu(s) aq) - Cu*²(aq) + 2 + 2 Fe*3, Fe+2, "(aq) Write the CAPITAL LETTER of the best answer. (A) Cu*2|Cu||Fe*3|Fe*2 (B) Fe*3|Fe*2||Cu*2|Cu (C) Cu*2|Cu||Fe*2|Fe*3 (D) Cu|Cu*2||Fe*3|Fe*2 (E) Fe*3| Fe*2||Cu|Cu* +2 Answer:arrow_forward(7.11) A voltaic cell is constructed that uses the following reaction and operates at 298 K: Zn(s) + Cd+2, Cd*2(aq) ® + (S)uZ Zn*2(aq) + Cd(s) a) What is the emf of the cell under standard conditions? Zn*2 (ag) + 2e¯ ® Zn(s) E°cell = -0.76 V cd*2 (aq) + 2e¯ ® Cd(s) E°cell = -0.40 V Answer: The emf of the cell is V. Refer to the Table of Standard Reduction Potential below to solve Question (a). STANDARD REDUCTION POTENTIAL AT 25 °c E() Hall Reaction +0.N0 2 H,O) + ze Hg) + 2OH (a) Hal-Reaction Hall-Reaction E(V) Ag"(ng) +e- Ag(s) --0.83 Aglir(s) +e- Ag(s) + Br (ag) AgCKs) +e- Ag(s) + a"(a) Ag(CN), "(a) +e Ag(s)+ 2CN (a) Ag:Cro(s) + 2e 2 Ag(s) + Cro (ag) +0.10 HO, (a) + H,O) + 2e 3OH (ay) +0.22 H,O(ng) + 2H"(ag) + 2e 2 H;O() -0.31 Hg/ (a) + 2e 2 Hg() +0.45 2g"(ay) + 2e 1Hg (a) -0.15 Hg" (a) + 2e - Hg() +0.01 s) + 2e 21(a) +1.78 +0.79 +0.92 Agl(s) +e- Ag(s) +I(ay) +0.85 Ag(S0,) (ay) +e Ag(s) + 2 S,0 (ap) +0.54 A (ay) + 3e Al(s) HAso,(a) + 2H'(ay) + 2e - HAsOdany) + HO() Ba"(ag) + 2e -…arrow_forward(7.4) Describe in shorthand notation a galvanic cell for which the cell has the following reaction. Chose the BEST option by writing the CAPITAL LETTER of the best answer. Cu(s) + 2 Fe*3(aq) - Cu*?(aq) + 2 (be). Fe2 (aq) CHOICES: (A) Cu Cu*2||Fe*3|Fe*2 (B) Cu*2|Cu||Fe*3| Fe*2 (C) Cu|Cu*2||Fe*2 |Fe*3 (C) Cu*2|Cu||Fe*2| Fe*3 Answer:arrow_forward
- (7.15a) What mass of copper is plated out in the electrolysis of CuSO4, in the same time that it takes to deposit 1.00g of Ag in a silver coulometer that is arranged in series with CUSO4 cell? (7.15b) If a current of 1.00 A is used, how many minutes is required to plate out this quantity of copper? Molar Mass (g/mol): Cu 63.546 Ag 107.87 Analysis/Strategy: ampere & time coulombs ® mol e¨ (or Faraday) ® mol Cu mass of Cu time to plate out Cu = time to plate out 1.00 g Ag (in series with CUSO4 cell) Reduction half-reaction of silver metal: Ag"(aq) + e ® Ag(s) Reduction half-reaction of copper metal: Cu*²(aq) 1+2, + Cu(s)arrow_forward(7.15a) What mass of copper is plated out in the electrolysis of CUSO4, in the same time that it takes to deposit 1.00g of Ag in a silver coulometer that is arranged in series with CUSO4 cell? (7.15b) If a current of 1.00 A is used, how many minutes is required to plate out this quantity of copper? Molar Mass (g/mol): Cu 63.546 Ag 107.87 Analysis/Strategy: ampere & time coulombs ® mol e¯ (or Faraday) ® mol ® Cu ® mass of Cu time to plate out Cu = time to plate out 1.00 g Ag (in series with CuSO4 cell) Reduction half-reaction of silver metal: Ag*(aq) + e ® Ag(s) Reduction half-reaction of copper metal: Cu*2(aq) + e ® Cu(s) Answer: Mass of copper plated out = g (3 sig fig) Minutes required to plate out copper using 1.00 ampere = minutes (3 sig fig)arrow_forwardQuestion 1.4 1.4 A metal cube has a total surface area of 690 cm2 and is to be gold-plated to a thickness of 0,5 cm. The process needs to be completed in exactly 2 hours. The electrochemical equivalent of gold is 1,118 mg/C and the density of gold is 19300 kg/m3. 1.4.1) Calculate the current required for the platingarrow_forward
- 9:53 X Electrochemistry ( due 11/16): Atte... 1. 6. What is E° cell for the following balanced reaction? Zn(s) + Pb2+(aq) → Zn2+(aq) + Pb(s) Half-reaction Standard Reduction Potential. Show your work. (10) 1. 2. 763 3. 126 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 7. The standard reduction potentials are +0.80 V for Ag+ on the reduction potentials, draw a diagram of the two half cells where the reduction and -0.76 V for Zn2+ . Based and oxidation will occur. Write down the two half-cell reactions. (10+10) Save and Close Submitarrow_forwardComplete and balance the following redox reaction in basic solution Zn(s) → Zn(OH),*(aq) + H,(g) 4 4- 2- D2+ 3+ 4+ + 1 2 3 4 6. 7 8 9. O3 O6 O7 (s) (1) (g) (aq) + e Zn H* OH Reset • x H,0 Delete 9. 4. 3. 2.arrow_forward(7: 4 pts) Balance the following redox reaction in basic conditions. Show your work for partial credit.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY