
Cyclobutadiene and (Cyclobutadiene)tricarbonyliron
As we saw in Section
12.17, cyclobutadiene is antiaromatic and exceedingly difficult to prepare and study. Its successful preparation by Rowland Pettit (University of Texas) in
1965 demonstrated how
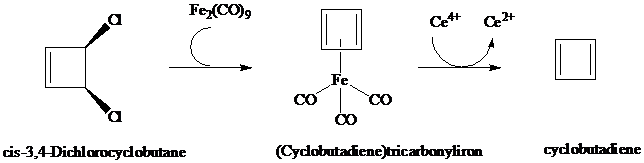
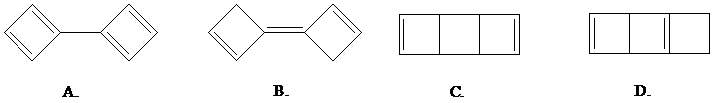
Once freed from its iron tricarbonyl complex, cyclobutadiene is unstable and dimerizes
readily. The structure of the dimer is:
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry - Standalone book
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


